- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2010
- Soft condensed matter
- Crucial roles of charged saccharides in the survival of Gram-negative bacteria
Crucial roles of charged saccharides in the survival of Gram-negative bacteria
The outer surface of Gram-negative bacteria displays a dense layer of saccharide chains connected to lipids, called lipopolysaccharides (LPSs). Since the removal or mutation of LPSs is known to result in the death of Gram-negative bacteria, LPSs are supposed to play crucial roles in the survival of bacteria against antimicrobial peptides. This finding inspires the design of various antibacterial compounds that primarily target LPSs. For example, cationic antimicrobial peptides (CAPs) have drawn increasing attention as alternatives to chemical food preservatives and commonly used antibiotics. To date, many in vivo studies demonstrated that divalent cations (Ca2+, Mg2+) significantly increase the survival rate of bacteria. However, the structural evidence from experimental approaches at the molecular level is still missing.
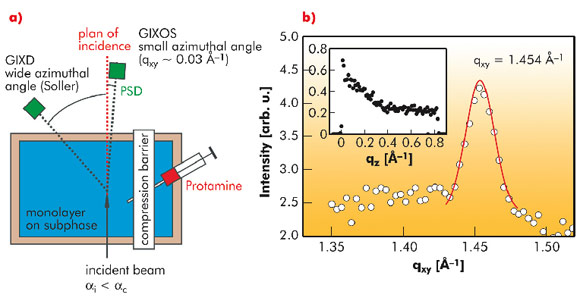
Here we reveal the influence of molecular structure (genetic mutation) and divalent cations on the survival of Gram-negative bacteria against cationic peptides like protamine. As the minimal experimental model of outer membranes of Gram-negative bacteria, we prepared the monolayers of LPSs purified from various bacterial strains at the air/water interface. The impact of mono- and di-valent ions and protamines on the fine structures of model membranes were probed by grazing-incidence X-ray diffraction (GIXD) and grazing-incidence X-ray scattering out of the specular plane (GIXOS) experiments at beamline ID10B (Figure 62a). GIXD enables one to gain the lateral ordering of hydrocarbon chains in the membrane plane, while GIXOS allows the determination of vertical electron density profiles perpendicular to the membrane plane.
Figure 62b shows the intensity integrated along qz plotted as a function of qxy for a deep rough mutant lipopolysaccharide (LPS Re) monolayer on a Ca2+-loaded subphase. The measured GIXD signal exhibits a single peak with its maxima located at qz = 0, indicating that the hydrocarbon chains of LPS Re molecules take an upright conformation and form a hexagonal lattice. In contrast, the GIXD signal measured at the same area per molecule showed no detectable peak in the absence of Ca2+, suggesting that Ca2+ also increases the lateral ordering of hydrocarbon chains. This finding was supported by coarse-grained Monte Carlo (MC) simulations, suggesting that the LPS headgroups become more compact in the presence of Ca2+.
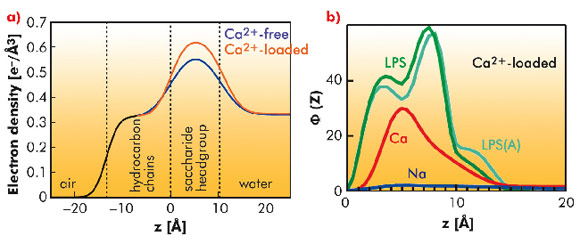
GIXOS measured at an azimuthal angle near the incidence plane can be translated to the corresponding reflectivity curve within a significantly reduced radiation time (by a factor of 100). Figure 63a shows the reconstructed electron density profiles of the LPS Re monolayers on Ca2+-free (blue) and Ca2+-loaded (red) subphases, indicating that Ca2+ induces an increase in headgroup electron density and/or a reduction in the roughness of the headgroup/water interface. MC simulations supported the experimental finding, suggesting that Na+ ions in the charged saccharide region (called the Kdo core) are displaced in the presence of Ca2+ ions (Figure 63b). When protamine is injected beyond the survival level of bacteria, we observed the complete destruction of the LPS Re monolayers in the absence of Ca2+. In contrast, the film remained completely intact in the presence of Ca2+, suggesting that Ca2+ accumulated near the Kdo core generate an electrostatic barrier.
To highlight the crucial role of charged saccharides, we carried out the same series of experiments with the acid hydrolysis product without the Kdo core (Lipid A). The Lipid A monolayer is different from the LPS Re monolayer in that it does not stay fully intact against protamine even in the presence of Ca2+, suggesting the crucial role of the Kdo core in bacterial survival. This finding agrees well with the fact that Lipid A is not sufficient for the growth of most of Gram-negative bacteria.
Authors
E. Schneck (a), R. Oliveira (b), D. Pink (c), O. Konovalov (d) and M. Tanaka (a).
(a) University of Heidelberg (Germany)
(b) University of Cordoba (Argentina)
(c) St. Francis Xavier University (Canada)
(d) ESRF
References
R. Oliveira et al., Phys. Rev. E 81, 041901 (2010).
E. Schneck et al., Proc. Natl. Acad. Sci. 107, 9147 (2010).
R. Oliveira et al., C. R. Chimie 12, 209 (2009).
E. Schneck et al., J. R. Soc. Interf. 6, S671 (2009).