- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Structure of materials
- 3D Ordered gold strings by coating nanoparticles with mesogens
3D Ordered gold strings by coating nanoparticles with mesogens
Gold nanoparticles associate into highly-ordered structures when coated with ligands formed from mesogens, the rigid parts of a liquid-crystal molecule. The nanoparticles no longer follow the usual packing of spheres and form columnar structures, strings, with interparticle spacing that can be controlled by the choice of co-ligand.
Metallic nanoparticles have attracted great interest for their potential electronic [1], optical [2], photonic [3] and medical [4] applications. These applications normally require the fabrication of bulk 3-D arrays of metal nanoparticles, or “meta-atoms”, with prescribed geometrical layouts. So far most of these arrays could only be produced with exceptionally uniform nanoparticles, and the range of array types was limited by the particle shape; e.g. cubic arrays from spherical particles. Recently Mehl and Cseh managed to graft liquid crystal molecules side-on to the nanoparticles [5]. Following on from this work, we have created self-assembled 3-D and 2-D arrays of these liquid-crystal-coated gold nanoparticles.
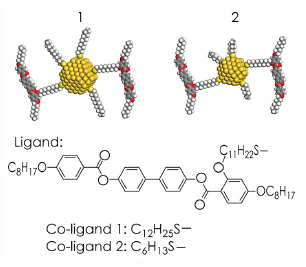
Two different mesogen-covered gold nanoparticle systems were studied, as shown in Figure 28. Small nanoparticles (ca. 2 nm in diameter) were used. Such materials preserve the ability of the mesogens to form a liquid crystalline phase. The alignment of the mesogens breaks the cubic symmetry that the nanoparticles would normally adopt, and introduces anisotropy to the periodic lattices that form.
 |
|
Fig. 28: Schematic structure of the gold nanoparticles coated with mesogens and alkylthiols. |
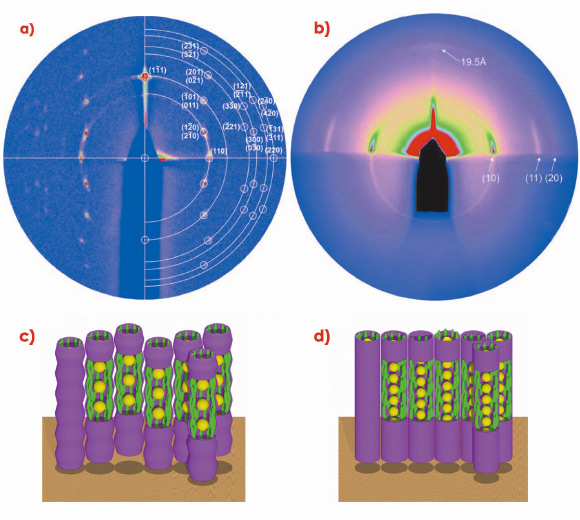
Grazing incidence small-angle X-ray scattering (GISAXS) experiments, carried out at beamline BM28 (XMaS), were extremely useful in determining the structures of these anisotropic arrays. GISAXS patterns from well-oriented films of systems 1 and 2, prepared on silicon substrate, are shown in Figures 29a and 29b. On the basis of these patterns, 1 was found to form a 3D rhombohedral lattice with space group R![]() m, while 2 was shown to organise into a 2D hexagonal columnar lattice. The schematic models of the two phases are shown in Figures 29c and 29d.
m, while 2 was shown to organise into a 2D hexagonal columnar lattice. The schematic models of the two phases are shown in Figures 29c and 29d.
GISAXS experiments on oriented films of 2 provided additional information about the columnar phases. Figure 29b shows that the nanoparticle columns stand perpendicular to the substrate surface. A weak diffuse meridional scattering peak indicates that the average distance between neighbouring gold nanoparticles within a column is just 2.0 nm.
The mesophase structure of both 1 and 2 can be described as consisting of strings of nanoparticles surrounded by a sheath of axially aligned mesogens. A significant feature illustrated by the present results is the remarkable scope for varying the interparticle distance in the column direction simply by varying the length of the co-ligand thioalkyl chains. Thus, while in 1 the interparticle gap is 1.7 nm, in 2 the gap virtually disappears and the nanoparticles touch in the direction of the string.
 |
|
Fig. 29: GISAXS diffraction patterns of a) system 1 and b) system 2, and corresponding schematic models of c) the rhombohedral phase in system 1; d) the hexagonal columnar phase in system 2; yellow: gold nanoparticles, green: mesogens. |
This work demonstrates that highly-ordered superlattices of metal nanoparticles other than those expected from mere packing of spheres can be created by coating the particles with liquid crystals. The results establish the first rules on which to base future designs of more complex lattices with a view to building self-assembled metamaterials.
References
[1] S.W. Boettcher, N.C. Strandwitz, M. Schierhorn, N. Lock, M.C. Lonergan and G.D. Stucky, Nat. Mater. 6, 592-596 (2007).
[2] S. Eustis, M.A. El-Sayed, Chem. Soc. Rev. 35, 209-217 (2006).
[3] P.N. Prasad, Nanophotonics, John Wiley & Sons, Inc. Hoboken, New Jersey (2004).
[4] J. Kim, Y. Piao, T. Hyeon, Chem. Soc. Rev. 38, 372-390 (2009).
[5] L. Cseh, G.H. Mehl, J. Mater. Chem. 17, 311-315(2007).
Principal publication and authors
X. Zeng (a), F. Liu (a), A.G. Fowler (a), G. Ungar (a), L. Cseh (b,d), G.H. Mehl (b) and J.E. Macdonald (c), Advanced Materials 21, 1746-1750 (2009).
(a) Department of Engineering Materials, University of Sheffield (UK)
(b) Department of Chemistry, University of Hull (UK)
(c) School of Physics and Astronomy, Cardiff University (UK)
(d) Present address: Romanian Academy, Institute for Chemistry, Timisoara (Romania)



