- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- Enabling Technologies
- Controlled dehydration of macromolecular crystals to improve diffraction properties
Controlled dehydration of macromolecular crystals to improve diffraction properties
The weakly diffracting nature of protein crystals often leads to data of insufficient quality to answer the biological question being asked, either due to a failure to solve the structure or to other factors, such as insufficient resolution to accurately determine modes of ligand binding. Methods exist to improve the diffraction properties of macromolecular crystals, dehydration often being found the most effective. Dehydration of protein crystals often has positive effects: reductions in the length of one or more unit cell dimensions are often accompanied by an increase in diffraction data resolution; a decrease in the mosaic spread and improvement in the profile of Bragg peaks. However, despite many descriptions of successful dehydration experiments and the availability of dedicated systems, the technique remains little used as conditions can be difficult to reproduce and the method can be problematic to implement. To standardise the technique, the EMBL and ESRF developed a humidity control device (HC1, Figure 148). Based on a modified cryostream nozzle, it produces an air stream at the sample position and allows precise control of the relative humidity (RH) between 50 and 99%. The device is fully compatible with the standard experimental environment at the ESRF and coupled with a synchrotron beamline, dehydration experiments have now become a practical way to improve the diffraction properties of some protein crystals.
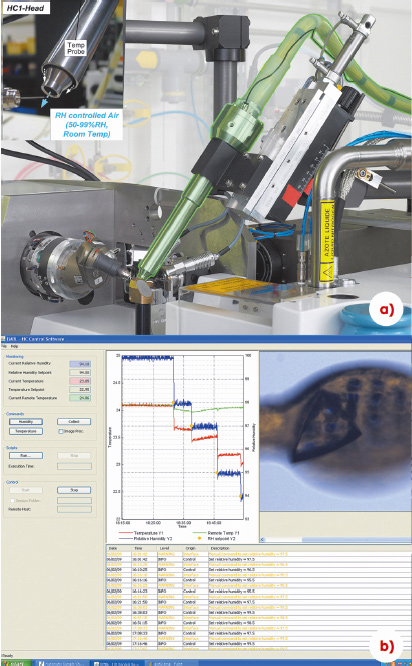 |
|
Fig. 148: a) The HC1b humidity control device mounted on an ESRF MX-Beamline. b) The HC1b GUI, a RH step gradient and a crystal being conditioned are shown. |
The device was initially tested using bovine mitochondrial F1-ATPase. This complex crystallises in the orthorhombic space group P 212121 with unit cell dimensions of a = 108, b = 140 and c = 285 Å. Crystals of F1-ATPase are known to react well to dehydration. Indeed, they are inclined to undergo spontaneous dehydration events, where the c cell dimension is reduced to ca 268 Å, after increasing the precipitant concentration or during prolonged crystal handling. This change in unit cell dimensions occurs rapidly and is favourable as it leads to an increase in intermolecular contacts within the crystal [1]. Further shrinkage of the unit cell dimensions has only been observed using controlled dehydration. Using the HC1b device it was possible to identify six ‘stable’ states in the dehydration pathway. The collection of low resolution data sets, at room temperature, allowed the full characterisation of these states and identified changes that occur only during dehydration. The six states were initially defined by diffraction patterns (Figure 149) containing well defined Bragg peaks from a single lattice with distinct unit cell parameters (if states were not stable, Bragg peaks were often split and multiple lattices existed). The final state, at an RH of 96%, diffracts to around 1 Å better than the more hydrated crystals. Once cryo-cooled, crystals in this final state have lost 20% of their unit cell volume, the number of intermolecular contacts has increased twenty-fold and they diffract X-rays to a resolution better than 2 Å, a remarkable diffraction limit for such a large complex.
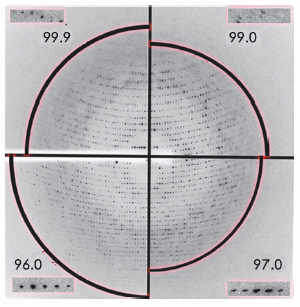 |
|
Fig. 149: Changes in X-ray diffraction of an F1-ATPase crystal during dehydration in 4 steps. Each of the four quadrants of the image show the diffraction pattern at each stable dehydration stage (where the arc of the circle is at the following resolutions: 3 Å, 3.8 Å, 4 Å and 2.5 Å). The inserts show a magnified view of the same area on the detector, demonstrating the improvement in Bragg peak profile after dehydration. |
The device has recently been made available to the ESRF user community and some initial successes include the chromatin remodelling complex (an improvement in resolution limit from 8 Å to 2.9 Å) and the Na+-translocating NADH:quinone oxidoreductase (NQR) from Vibrio cholerae (an improvement in resolution limit from 6 Å to 4 Å). A particularly significant example is with crystals of plant photosystem I that have been observed to undergo a transition between their starting RH of 99% and 97%. An improvement in diffraction limit from around 6 Å to 4 Å is seen which is accompanied by visual improvements in diffraction quality. Currently, the highest resolution data measured from cryo-cooled crystals of this 530 kDa integral membrane protein is 3.4 Å. The dehydrated crystals have the potential to yield data to a significantly increased resolution. Such data would provide improved mechanistic details for this important complex.
As more examples are found, and as more devices are commissioned at other synchrotrons (Diamond, CLS and Max-Lab), it is hoped that general rules on dehydration protocols can be developed. The device also has promise for the collection of higher resolution data from non-cryo-cooled crystals (a new version, the HC2, will control the temperature of the air stream to 5 - 7°C) and for the on-line exchange of water in macromolecular crystals with deuterated water for neutron diffraction experiments.
Reference
[1] M.W. Bowler, M.G. Montgomery, A.G. Leslie and J.E. Walker, Acta Cryst D 62, 991-995 (2006).
Principal publication and authors
J. Sanchez-Weatherby (a), M.W. Bowler (b), J. Huet (a), A. Gobbo (a), F. Felisaz (a), B. Lavault (a), R. Moya (a), J. Kadlec (a), R.B.G. Ravelli (c) and F. Cipriani (a), Acta Cryst. D. 65, 1237-1246 (2009).
(a) EMBL Outstation at Grenoble (France)
(b) ESRF
(c) Leiden University Medical Center (The Netherlands)



