- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- Surface and interface science
- The shape of supported rhodium nanoparticles during oxidation and reduction
The shape of supported rhodium nanoparticles during oxidation and reduction
The world market for the production of chemicals is estimated today to be around 1,300 billion Euros per year. More than 90% of these chemicals are produced via a catalytic process.
Catalysts also play an increasingly important role for the development of green chemistry and advanced renewable energy technologies.
A study on catalytic nanoparticles carried out on beamline BM32 gives an example of how in situ X-ray scattering in dedicated sample environments can bridge the gap between structural and functional properties of nanostructures. In particular the interplay between the shape and size change of the nanoparticles and the oxidation/reduction process can be monitored.
The current challenge for fundamental research is to provide a detailed microscopic understanding of the different physical and chemical processes which take place at nanoparticles during catalytic reactions [1]. It is expected that nanoparticles should exhibit enhanced catalytic activity: (i) as they possess an increased number of under-coordinated atoms, and (ii) as different low index facets co-exist, facilitating mass transport and thereby lifting kinetic barriers known from single crystal surfaces. During catalytic cycling experiments, nanoparticles have been observed to undergo reversible size changes, which are associated with cyclic shape changes, material re-dispersion and sintering.
Here, we report an in situ high-resolution X-ray diffraction study of epitaxial rhodium nanoparticles on MgO(100), during oxidation and CO induced reduction. This study brought to light a reversible facet rearrangement of the nanoparticles, which is in direct relation to the formation of oxygen-induced superstructures. We were able to access the average particle shape and size with atomic resolution from a quantitative fitting of the extended reciprocal space maps that were obtained at elevated temperatures and under varying gas atmospheres.
Rhodium nanoparticles were deposited in situ at a substrate temperature of 670 K in the BM32 ultra-high vacuum (UHV) surface X-ray diffraction chamber, after cleaning the MgO substrates by sputtering and annealing under an oxygen atmosphere. The sample was annealed at 970 K to achieve the equilibrium shape of the nanoparticles (see Figure 94a). Grazing incidence small angle scattering was performed simultaneously to wide-angle diffraction reciprocal-space mapping.
 |
|
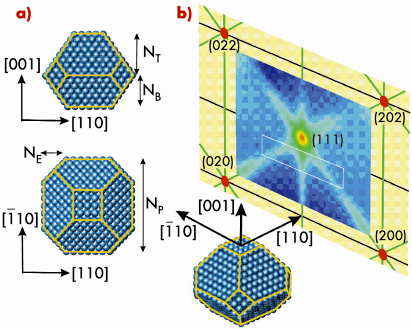
Fig. 94: a) Schematic representation of the particle shape for fcc based nanoparticles. The lateral particle size is characterised by afcc/2 *NP, its height by afcc /2 *(NT+NB) and the missing edge atoms by NE. For a Wulff shaped particle (without substrate) is NT = NB and NE = NP-NT. b) (110) plane reciprocal space map of clean Rh nanoparticles on MgO(100) with an average lateral size of 8 nm (in fcc bulk coordinates). The white box indicates the area for high resolution scans. |
High-resolution reciprocal space maps were recorded from (H,K) = (-0.5,0.5) to (0.5,-0.5) and from L = 0.6 to 0.84. Figure 95a (top) shows a high-resolution map of the clean particles, observed at 600 K under UHV conditions. The data can be understood in a straight forward way within kinematical diffraction theory which suggests nanoparticles with a truncated octahedral shape.
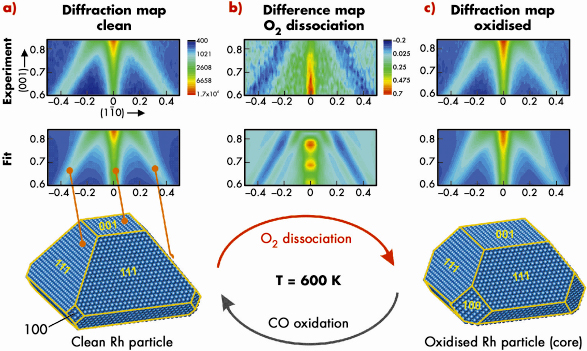
In the next step, the Rh nanoparticles are exposed to 3 x 10–5 mbar O2 at 600 K and simultaneously we recorded the X-ray diffraction pattern (Figure 95 (b and c)). We actually observe a very clear change in the X-ray diffraction signal (see difference map, Figure 95b) which consists of an intensity enhancement along the (001) rod and an intensity loss along the (111)-rods.
 |
|
Fig. 95: a) (top) (110) Diffraction map of clean Rh particles at 600 K. (middle) Fitted diffraction map corresponding to the average particle shape given below. b) (top) Oxygen induced signal change in the (110) plane. (middle) Simulated signal change for particles with increased (100) side facet area. c) (top) Experimental (110) diffraction map at 600 K and 3 x 10–5 mbar O2 pressure. (middle) Fitted diffraction map for particles under oxygen exposure. (bottom) Best fit core particle shape after oxidation. |
As a result of this analysis we find that the area both of the (100) side facets and (001) top facet increases upon oxidation, which is driven by the formation of ultrathin oxide layers. This implies that only intra particle mass transport takes place during the oxidation thereby removing Rh atoms from the (100) side and top facets. The average amount of atoms removed corresponds to the number of atoms incorporated into the surface oxide layers on all facets. A further key observation is that the oxygen-induced shape change of the Rh nanoparticle is fully reversible when the surface oxide is removed by CO exposure (at 1 x 10–5 mbar). We have demonstrated that in situ reciprocal space mapping can give an atomistic insight into the structure and morphology of catalytically-active nanoparticles on oxide supports.
Principal publication and authors
P. Nolte (a), A. Stierle (a), N.Y. Jin-Phillipp (a), N. Kasper (a), T.U. Schülli (b), H. Dosch (a), Science 321, 1654 (2008).
(a) Max-Planck-Institut für Metallforschung, Stuttgart (Germany)
(b) INAC/ SP2M Commissariat àl’Energie Atomique, Grenoble (France)
Reference
[1] G. Ertl, H. Knözinger, F. Schüth, J. Weitkamp, Handbook of Heterogeneous Catalysis, Wiley-VCH, Weinheim (2008).



