- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- Materials science
- Hydrogen-induced Ostwald ripening
Hydrogen-induced Ostwald ripening
The main aim in hydrogen storage research is to obtain high hydrogen concentrations in a material that possesses suitable transport properties under ambient conditions. A promising way to favourably alter the material’s properties is by reducing its size to the extent that surface and quantum effects begin to play a major role. The investigation of nanocluster metal hydrides may therefore reveal novel properties. Palladium is one of the most widely studied metals with respect to hydrogen absorption. At elevated temperatures palladium nanoclusters change size due to Ostwald ripening: the larger clusters capture mobile atoms at the expense of smaller clusters [1]. As hydrogen in a metal can decrease the strength of the host metal bonding, this raises the question of how an ensemble of palladium nanoclusters will interact during and after hydrogenation. A morphological or structural change of the nanocluster ensemble may affect the hydrogenation properties as compared to a single cluster.
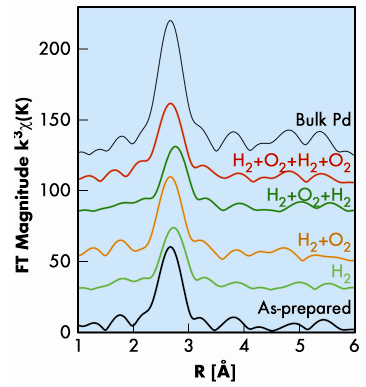 |
|
Fig. 23: Phase-corrected Fourier transforms of the k3-weighted Pd nanocluster EXAFS as a function of the atomic distance (R). |
We investigated the effect of hydrogen exposure on a palladium cluster assembled film produced with a dual-target dual-laser vaporisation source with three different techniques: extended X-ray absorption fine structure (EXAFS), X-ray diffraction (XRD) and scanning-tunnelling microscopy (STM). These three complementary methods were used to determine the size changes of the palladium nanoclusters upon exposure to hydrogen and oxygen. The average grain size was derived both from the coordination number around palladium atoms obtained from EXAFS (Figure 23) and the width of the XRD Bragg peaks, whereas the (lateral) diameter of the nanoclusters at the sample surface was measured directly by STM. X-ray absorption data (Figure 23) collected at the DUBBLE beamline (BM26A) yielded additional parameters such as inter-atomic distances, degree of disorder and electronic information through the shift of the absorption edge; these support the observation of size changes. Upon hydrogenation, the increase in the nanocluster size measured with XRD, EXAFS, and STM is 22%, 38%, and 37%, respectively. This increase is much larger than the increase of about 8.1% in the Pd phase unit cell volume corresponding to the hydride formation. The cluster growth is due to an atomic reorganisation by three-dimensional Ostwald ripening, in which the larger clusters take up mobile atoms at the expense of smaller clusters [1]. For most materials, spontaneous Ostwald ripening is an extremely slow process at room temperature. However, the presence of a hydrogen atom in the metal reduces the binding energy, thus increasing the probability of detachment of palladium atoms as shown schematically in Figure 24. This process can be qualitatively understood in terms of the difference between the sublimation energy of palladium metal and palladium hydride. The sublimation energy of palladium decreases with increasing hydrogen concentration and is about 50% lower at a H/Pd ratio of 0.3 [2]. The effect of hydrogen on the palladium cluster sublimation has the same result as if the temperature of the clusters was doubled. Since our experiment was performed at room temperature, a doubled temperature would correspond to approximately 600 K (327ºC), a temperature at which Ostwald ripening would occur. Thus the observed cluster size increase is attributed to Ostwald ripening induced by exposure to hydrogen: the absorbed hydrogen decreases the sublimation energy of palladium, which stimulates the detachment of atoms from the clusters at room temperature.
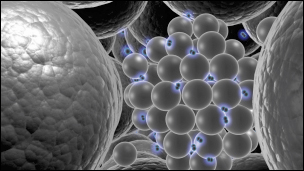 |
|
Fig. 24: A schematic impression of the palladium cluster building blocks. The palladium atoms become free to move due to the interstitial hydrogen. |
To conclude, the morphological and structural changes occurring in an ensemble of palladium nanoclusters have been studied after several hydrogenation cycles with EXAFS, XRD, and STM. Initial hydrogenation increased the cluster size, a result that is attributed to hydrogen-induced Ostwald ripening. This phenomenon originates from the higher mobility of palladium atoms resulting from the low sublimation energy of palladium hydride as compared to that of the palladium metal. The universality of this phenomenon makes it important for the application of future nanostructured hydrogen storage materials.
Principal publication and authors
M. Di Vece (a), D. Grandjean (a), M.J. Van Bael (a), C.P. Romero (a), X. Wang (a), S. Decoster (b), A. Vantomme (b) and P. Lievens (a), Phys. Rev. Lett. 100, 236105 (2008).
(a) Laboratorium voor Vaste-Stoffysica en Magnetisme & INPAC - Institute for Nanoscale Physics and Chemistry, K.U. Leuven (Belgium)
(b) Instituut voor Kern- en Stralingsfysica & INPAC - Institute for Nanoscale Physics and Chemistry, K.U. Leuven (Belgium)
References
[1] W. Ostwald, Z. Phys. Chem. (Leipzig) 34, 495 (1900).
[2] N.V. Piskunov et al., J. Eng. Phys. Thermophys. 74, 1217 (2001).



