- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- Structural biology
- Pilus assembly across the bacterial outer membrane
Pilus assembly across the bacterial outer membrane
The chaperone-usher (CU) pathway is a translocation system used to assemble adhesive multi-subunit fibres on the outer surface of gram-negative bacteria. CU pili are formed by the non-covalent polymerisation of several hundreds or thousands of pilus subunits which consist of an incomplete immunoglobulin (Ig)-like fold lacking the C-terminal ß-strand. In the periplasm, a cognate chaperone assists in pilus subunit folding by donating a b-strand to complement the truncated Ig-like fold of the pilus subunit, a process termed donor-strand complementation (Figure 69, A*) [1]. Chaperone:subunit complexes are then recruited to a pilus assembly platform in the outer membrane (OM) called the “usher”. The usher catalyses ordered subunit polymerisation and mediates translocation of the nascent pilus to the cell surface. Polymerisation of pilus subunits occurs through an intermolecular fold complementation mechanism involving the first 10-20 residues (termed “N-terminal extension” or Nte) of the pilus subunit next in assembly. The Nte of a newly recruited subunit inserts in the groove (left by the missing strand) of the subunit previously assembled, competing off the chaperone still associated with that subunit (Figure 69, B*) [2]. This so-called “donor-strand exchange” process, in which the chaperone donor-strand is exchanged for an incoming subunit’s Nte, occurs in the absence of an electrochemical gradient or an hydrolysable energy source. How subunit polymerisation and fibre translocation across the membrane are orchestrated by the usher remains poorly understood, largely due to the lack of high resolution structural information on the assembly platform.
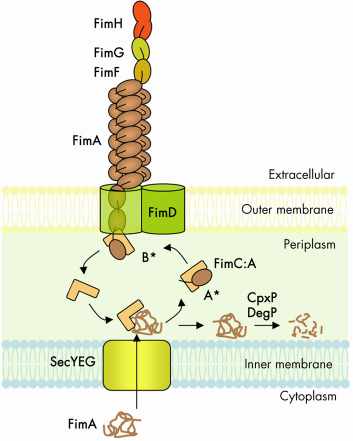 |
|
Fig. 69: Schematic representation of the CU biosynthetic pathway, labelled according the type 1 pilus system (Fim cluster). |
Using data collected at the beamlines ID29 and ID23-1, we determined the crystal structure of the detergent-solubilised translocation domain of the P pilus usher PapC to 3.2 Å resolution. Ushers are ~800 residue proteins comprising a central β-barrel domain flanked by periplasmic soluble domains of ~125 and ~170 residues at the N- and C-termini respectively. The N-terminal domain forms the recruitment site for chaperone:subunit complexes. The function of the C-terminal domain is unknown, though some indications point to a possible role in an early activation event. The crystal structure reveals the usher translocation pore comprises a 24-stranded ß-barrel that gives rise to a channel with inner diameter of ~ 45 by 25 Å, compatible with the translocation of folded pilus subunits (Figure 70). In its non-activated form, however, this pore is occluded by a folded plug domain that is inserted into the loop between strands 6 and 7. In addition, a b-hairpin formed by adjacent strands curves back out of the barrel wall, into the pore lumen and contacts the base of the plug domain. Most likely usher activation necessitates a conformational change in these two elements, resulting in opening of the pore. The crystals of the PapC translocation domain also exhibit a dimerisation interface along the flat surface of the kidney-shaped β-barrel, very similar to the twinned pore observed by electron microscopy of 2D crystals of lipid-reconstituted full-length PapC.
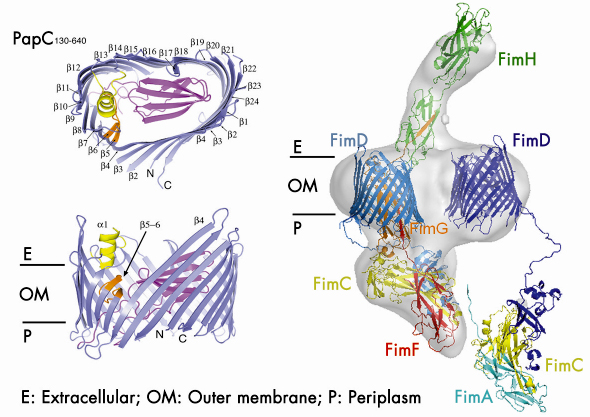 |
|
Fig. 70: (Left) Side and top views of the PapC translocation channel. (Right) Model of the type 1 pilus assembly intermediate (FimD2:FimC:FimF:FimG:FimH) docked into the reconstructed electron density obtained with single particle cryo-EM. A newly incoming chaperone:subunit complex (FimC:FimA) is modelled in, bound to the N-terminal domain of the second usher (FimD). |
In combination with the single particle cryo-electron microscopy imaging of the type 1 pilus usher (FimD) bound to a translocating three-subunit assembly intermediate (Figure 70), this structure provides, for the first time, a molecular snapshot of a twinned pore translocation machinery responsible for CU pilus assembly at the bacterial outer membrane. Unexpectedly, only a single pore is used for organelle secretion. The combined structures suggest a model in which both usher protomers are required for subunit polymerisation through a mechanism of alternating chaperone:subunit complex recruitment. At each step of the incorporation cycle, the N-terminal domain of one of the two pores is engaged in binding the chaperone:subunit complex at the base of the growing fibre, whilst the N-terminal domain of the opposing pore forms the docking site for a newly incoming chaperone:subunit complex. During donor-strand exchange, the chaperone at the now penultimate site in the fibre is released, thereby liberating that usher’s N-terminal domain for a new recruitment step. In this way, alternations in chaperone:subunit recruitment at either usher N-terminus allow the step-wise incorporation of new pilus subunits, with the growing fibre translocating through the activated channel in the twinned pore complex.
Principal publication and authors
H. Remaut (a), C. Tang (c), N.S. Henderson (d), J.S. Pinkner (e), T. Wang (c), S.J. Hultgren (e), D.G. Thanassi (d), G. Waksman (a), and H. Li (c), Cell 133, 640 (2008).
(a) Institute of Structural and Molecular Biology, Birkbeck College/UCL London (UK)
(c) Biology Department, BNL, NY (USA)
(d) CID, Stony Brook University, NY (USA)
(e) DMM, Washington University School of Medicine, MO (USA)
References
[1] F.G. Sauer, K. Fütterer, J.S. Pinkner, K.W. Dodson, S.J. Hultgren and G.Waksman, Science 285, 1058 (1999).
[2] H. Remaut, R.J. Rose, T.J. Hannan, S.J. Hultgren, S.E. Radford, A.E. Ashcroft, and G. Waksman, Molecular Cell 22, 831 (2006).



