- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2008
- X-ray absorption and magnetic scattering
- In situ redispersion of platinum nanoparticles on ceria-based oxide for vehicle exhaust catalysts
In situ redispersion of platinum nanoparticles on ceria-based oxide for vehicle exhaust catalysts
Supported precious metals are used to facilitate many industrial catalytic processes. Platinum (Pt) in particular is used for the cleaning up of vehicle exhaust emissions. When the vehicle exhaust catalyst is exposed to high temperatures (~800°C and above), the highly dispersed metal nanoparticles agglomerate and sinter, decreasing the active surface area, which causes a loss of catalytic activity (i.e. deactivation). Exhaust gases exiting from gasoline engines change quickly during operation. Temperatures can rise transiently to around 1000°C and the exhaust gas composition itself fluctuates quickly between oxidative and reductive compositions. Hence, in situ dynamic observation on the sintering and redispersion phenomena of the precious metal in the automotive catalysts is very important indeed.
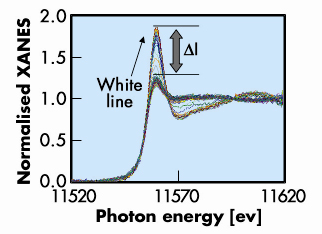
Real-time observation of the sintering/redispersion behaviour of Pt was made possible by the fluorescence yield variant (Turbo-XAS) of energy dispersive XAFS developed by Pascarelli et al. at ID24 [1]. An in situ cell optimised for fluorescence detection was designed for this experiment [2]. Fluorescence yield Turbo-XAS data collected at the Pt LIII edge on 2 wt% Pt/Ce-Zr-Y mixed oxide (referred to as CZY) catalysts under in situ conditions are shown in Figure 119. The signal to noise ratio on this data is improved with respect to our previous transmission XAS study on Pt/CZY, which showed that the combination of low levels of Pt in the catalysts, with high levels of heavy, absorbing, elements such as Ce and Zr severely compromises the conventional, transmission based experiments, making quantitative analysis very difficult [3].
 |
|
Fig. 119: Serial time-resolved Pt LIII edge XANES spectra (6 seconds each) of 2 wt% Pt/CZY catalyst under cyclical oxidising/reducing. Variation in the first hundred spectra under oxidising/reducing atmosphere at around 400ºC. |
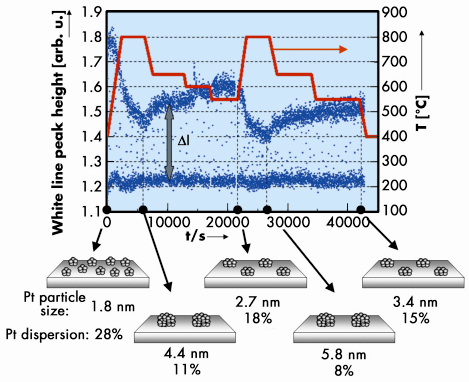
Figure 120 shows the variation of the white line peak height of the normalised XANES for the fresh Pt/CZY catalysts under cyclical oxidising/reducing condition at 400 ~ 800°C. ![]() I in Figure 120 denotes the difference between the white line peak height of the oxidised and reduced samples. From a previous experiment [3], we found that the
I in Figure 120 denotes the difference between the white line peak height of the oxidised and reduced samples. From a previous experiment [3], we found that the ![]() I increased with decreasing Pt particle size. Using the correlation between the Pt particle size and
I increased with decreasing Pt particle size. Using the correlation between the Pt particle size and ![]() I, we observed that the Pt particle size changed with temperature. The Pt particle size of the fresh catalyst increases from 1.8 to 4.4 nm while temperature is increased from 400 to 800°C, but then decreases to 2.7 nm as temperature is lowered to 550°C. It is particularly interesting to note that the sintering/redispersion phenomena occur reversibly and can be controlled using the temperature. During the redispersion process, the Pt particle size was recovered from 4.4 to 2.7 nm at first, and then from 5.8 to 3.4 nm. In contrast, only facile sintering of Pt particles occurs in alumina supported Pt catalysts (Pt/Al2O3), and Pt redispersion is not achieved at any temperature. Therefore, the Pt redispersion on CZY can be reasonably attributed to the strong Pt-ceria support interaction [4]. Through further kinetic measurements, using XAS and in situ transmission electron microscopy, an atomic migration mechanism is shown to account for the observed redispersion through the trapping of atomic Pt species at sites on the Ce support that exhibit a strong Pt-oxide-support interaction.
I, we observed that the Pt particle size changed with temperature. The Pt particle size of the fresh catalyst increases from 1.8 to 4.4 nm while temperature is increased from 400 to 800°C, but then decreases to 2.7 nm as temperature is lowered to 550°C. It is particularly interesting to note that the sintering/redispersion phenomena occur reversibly and can be controlled using the temperature. During the redispersion process, the Pt particle size was recovered from 4.4 to 2.7 nm at first, and then from 5.8 to 3.4 nm. In contrast, only facile sintering of Pt particles occurs in alumina supported Pt catalysts (Pt/Al2O3), and Pt redispersion is not achieved at any temperature. Therefore, the Pt redispersion on CZY can be reasonably attributed to the strong Pt-ceria support interaction [4]. Through further kinetic measurements, using XAS and in situ transmission electron microscopy, an atomic migration mechanism is shown to account for the observed redispersion through the trapping of atomic Pt species at sites on the Ce support that exhibit a strong Pt-oxide-support interaction.
 |
|
Fig. 120: Temporal dependence of the white line peak height of the Pt LIII edge XANES for the fresh 2 wt% Pt/CZY catalysts under oxidising/reducing atmosphere at 400 ~ 800°C and the schematic representation of the sintering/redispersion behaviour. 4 or 20% O2/He gas and 3% H2/He gas were alternately introduced into the cell every 60 seconds throughout the measurement. |
In conclusion, we have made the first in situ time-resolved fluorescence XAS measurements from a dilute sample using the Turbo-XAS acquisition mode. This technique allowed us to shed light on catalytic processes on the second timescale for Pt supported on a ceria-based oxide. Analysis of the data led to the discovery of an unexpected phenomenon, i.e. an efficient oxidative redispersion of Pt nanoparticles during quick (~ 60 seconds) redox cycling. This redispersion process does lead to a tangible potential for incorporation into “on board” methodology for extending vehicle catalyst lifetime through curtailing or reversing the effects of metal sintering during operation.
Principal publication and authors
Y. Nagai (a), N. Hara (b), K. Dohmae (a), Y. Ikeda (b, g), N. Takagi (c, g), T. Tanabe (a), G. Guilera (d, h), S. Pascarelli (d), M.A. Newton (d), O. Kuno (e, i), H. Jiang (e), H. Shinjoh (a), S. Matsumoto (f), Angew. Chem Int. Ed. 47, 9303 (2008).
(a) TOYOTA Central R&D Labs., Inc., Aichi (Japan)
(b) TOYOTA Motor Europe Technical Centre, Zaventem (Belgium)
(c) TOYOTA Motor Corporation Higashi-Fuji Technical Center, Shizuoka (Japan)
(d) ESRF
(e)TOYOTA Motor Engineering & Manufacturing North America, Inc., Michigan (USA)
(f) TOYOTA Motor Corporation, Toyota (Japan)
(g) Current Address : (f)
(h) Current Address : ALBA-CELLS, Barcelona (Spain)
(i) Current Address: (c)
References
[1] S. Pascarelli, T. Neisius, S. De Panfilis, J. Synchrotron Rad. 6, 1044 (1999).
[2] G. Guilera, B. Gorges, S. Pascarelli, H. Vitoux, C. Prestipino, Y. Nagai, N. Hara, submitted to J. Synchrotron Rad.
[3] Y. Nagai, N. Takagi, Y. Ikeda, K. Dohmae, T. Tanabe, G. Guilera, S. Pascarelli, M.A. Newton, H. Shinjoh, S. Matsumoto, AIP conference proceedings. 882, 594 (2007).
[4] Y. Nagai, T. Hirabayashi, K Dohmae, N. Takagi, T. Minami, H. Shinjoh, S. Matsumoto, J. Catal. 242, 103 (2006).



