- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2006
- Structural Biology
- The ID23-2 Microfocus Beamline
The ID23-2 Microfocus Beamline
Users of Macromolecular Crystallography (MX) beamlines regularly analyse crystals that are smaller than 50 õm and the tendency is towards even smaller, micro-sized samples. Quite often there is a need to analyse multiple rather than single crystals. One of the reasons for this is that increasingly challenging projects may only give tiny crystals. Additionally, increasing competition among structural biology groups demands a reduction in the time from project inception to structure publication and adequate time to optimise the production of crystals is not always available. To enable the MX user community to collect good quality data from such samples, synchrotron sources around the world are opening beamlines with so-called microfocus X-ray beams. These beamlines, of which ID13 at the ESRF was one of the pioneers, allow the collection of diffraction data with the X-ray beam matched to micro-sized crystals and also allow the âÂÂsearchingâ of poor quality crystals to find a portion suitable for the collection of good quality diffraction data.
In July 2001, the ESRF took the decision to build ID23. This beamline has two end-stations that operate as independently from each other as is reasonably possible. ID23-1 is a fully tunable MAD beamline. ID23-2 is the first microfocus beamline fully dedicated to MX and builds on the experience gained at ID13. The central challenge for ID23-2 was to provide the MX user community with a beamsize smaller than 10 õm in diameter whilst keeping the same âÂÂeasy-to-useâ environment and reliability as the other ESRF MX beamlines (ID14, ID23-1, ID29).
The X-ray source for ID23 is a canted undulator system comprising two undulators with a radial separation of 1.5 mrad. A canted undulator design was chosen to enable the installation of two end-stations on a single straight section, whilst retaining a maximal independence of beamline operation. For microbeam work to be routine, the beamline must be highly stable. To help achieve this, the optical setup of ID23-2 is as simple as possible and is composed only of high power primary slits, a âÂÂsingle bounceâ silicon monochromator, a set of secondary slits and the Kirkpatrick-Baez (KB) focussing element. The KB architecture is an easy-to-use system providing fine focussing. The ID23-2 device, housed in a steel container under helium gas, is composed of two 300 mm long Pt coated Si mirrors positioned 2 m from the sample. This results in a beam size of 7.5 by 5.5 õm2 (FWHM). The user environment is that of a standard MX beamline comprising a single-axis minidiffractometer and sample changer in the ID23-2 experimental hutch, together with the ancillary cryostream, attenuators, shutter, etc.
 |
|
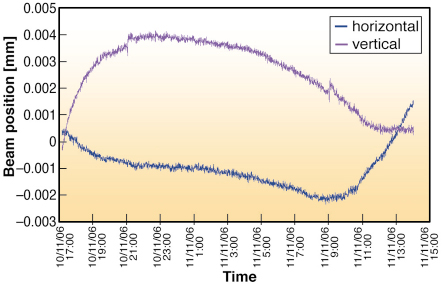
Fig. 72: The variation in vertical (magenta) and horizontal (blue) beam position on ID23-2 as a function of time. The slow drift observed is easily corrected before each data collection. |
Maintaining a beam focus of less than 10 õm, whilst keeping the beam centred precisely on the same section of a sample is challenging. At this scale, beam movements result from even small changes in ambient temperature. Indeed during initial beamline commissioning, beam drifts in the range of 15 õm over 12 hours were measured. Monitoring of the beam position and hutch temperature as a function of time showed that vertical beam drifts were correlated with a variation in the hutch temperature while horizontal drifts correlated with both hutch and monochromator motor temperature. Consequently, the KB chamber has been surrounded by a perspex box to improve its insulation from temperature changes. Beam drifts have been reduced to less than 5 õm over a 20 hour period (Figure 72) and we are working to reduce this further. To be 100% sure of the beam alignment, a pop-up fluorescent screen is available at the sample position allowing users to check the beam position before data collection starts. Beamline ID23-2 has been in standard operation for more than a year and has proved that microbeam X-ray diffraction can be made routine.
 |
|
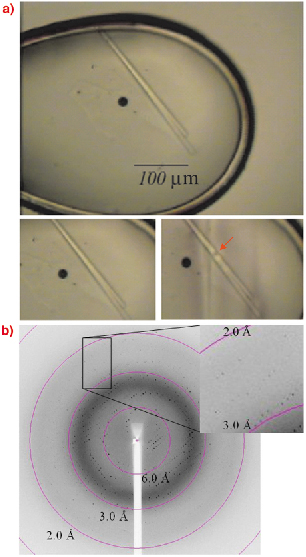
Fig. 73: The IREM -1 crystal (top) used for collecting the dataset described in the text. The diffraction pattern (bottom) was collected by exposing the portion of the crystal shown in the middle, right plate of this figure. |
Many projects have benefited from the availability of a microfocus beam. In one case, after many attempts, only long needle-shaped crystals of IREM 1 [1] could be obtained from crystallisation screening carried out at the High Throughput Crystallisation Laboratory (HTX Lab) of the EMBL Grenoble Outstation. Therefore, the scientists turned to ID23-2 to collect X-ray data from this single long needle crystal, which was 300 by 10 micrometres in size (Figure 73). Due to the high intensity of the ID23-2 X-ray beam, radiation damage was evident after only a few degrees of data had been collected, hence successive partial datasets were collected from four different sections of the same crystal and subsequently merged. This made it possible to obtain the crystal structure of the IREM-1 extracellular domain to 2.6 ÃÂ resolution [2].
References
[1] The inhibitory receptors expressed on myeloid cells (IREM) are type I transmembrane proteins encoded on human chromosome 17 (17q25.1), whose function is believed to be important in controlling inflammation. IREM-1 functions as inhibitory receptor, whereas IREM-2 and IREM-3 serve an activating function.
[2] Dimasi et al., Acta F, submitted.
Authors
D. Flot (a), T. Mairs (b), T. Giraud (b), M. Guijarro (b), V. Rey (b), D. van Brussel (b), P. Fajardo (b), O. Hignette (b), J. Chavanne (b), J.-C. Biasci (b), S. McSweeney (b), E. Mitchell (b).
(a) EMBL Grenoble Outstation (France)
(b) ESRF



