- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2004
- Materials Science
- Photodissociation of C2H4I2 Studied by Picosecond X-ray Diffraction
Photodissociation of C2H4I2 Studied by Picosecond X-ray Diffraction
How does the structure of a molecule change during a chemical reaction and how does the molecule interact with its nearest neighbours during the reaction? This is a very difficult question since most molecules are formed and broken extremely quickly. In fact the primary step in the formation of a molecule is the creation or breakage of chemical bonds or the transfer of electrons or protons. These steps are typically completed in 10-1000 femtoseconds, which is about 1000 times faster than the X-ray pulse from a synchrotron. A newly-formed molecule is usually created in a high-energy state, which relaxes into secondary structures by exploring all internal degrees of freedom in the molecule (turn angles, bond angles, bond lengths, etc). In this process, the molecule may reach a local minimum on the potential energy surface and later de-excite through collisions with its neighbours. These secondary structures, which typically have picosecond lifetimes in liquids, can be determined by single pulse X-ray diffraction from photosensitive molecules that can be activated by a short laser pulse. Here we report on the photo detachment of I2 from diiodoethane C2H4I2, see Figure 22. We now know that molecular iodine I2 is formed in a two-step process, which involves the unstable C2H4I radical:
C2H4I2 + h![]()
![]() C2H4I + I
C2H4I + I ![]() C2H4 + I2
C2H4 + I2
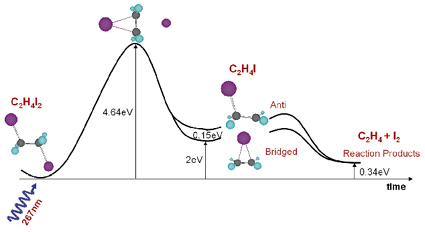 |
Fig. 22: Calculated energy landscape of the photoproducts of C2H4I2. Iodine is pink, carbon gray and hydrogen blue. |
Note that quantum chemistry predicts two intermediate structures of C2H4I in methanol (CH3OH) at nearly the same energy, the bridged and the anti structure. The experiment, which was done on beamline ID9B, shows that the bridged structure alone is formed in methanol, a fact that we ascribe to nearest neighbour interaction in liquid methanol.
The C2H4I2 molecules were dissolved at a concentration of 1:400. The C2H4I2 molecule is excited by the absorption of a UV pulse (2 ps, 267 nm) in the C-I stretched band, which leads to the emission of an iodine atom into the solvent. In 60% of the excited molecules, the freed iodine atom thermalises in the first solvation shell and recombines geminately. In 40% of cases, however, the C2H4I radical is formed and we would like to determine its structure, lifetime (decay mechanism) and the liquid structure around it. In the experiment, the pulsed laser beam (2 ps, 267 nm, 986 Hz) and the pulsed X-ray beam (100 ps, 0.07 nm, 986 Hz) were focused onto a 0.3 mm thick sheet of liquid formed by a sapphire nozzle. By suitably adjusting the speed of the liquid, a fresh sample was injected into the beam every millisecond. The time resolution is then simply the delay between the pump and the probe. The diffracted beam was recorded on a CCD detector and the images were recorded in pairs with and without excitation. The white beam from the single-harmonic undulator U17 was used to compensate for the low frequency of the experiment. The X-ray beam was focused by a toroidal mirror to 0.10 x 0.05 mm2 and the flux was 5 x 1011 ph/s in a 2.5% bandwidth around 18.0 keV. The difference in the radial intensity ![]() S(q,
S(q, ![]() ), non excited excited, is oscillatory and strongly dependent on the time delay. The associated Fourier transform is a measure of the change in the atom-atom pair distribution functions gij(r) within the molecule [1,2], see Figure 23.
), non excited excited, is oscillatory and strongly dependent on the time delay. The associated Fourier transform is a measure of the change in the atom-atom pair distribution functions gij(r) within the molecule [1,2], see Figure 23.
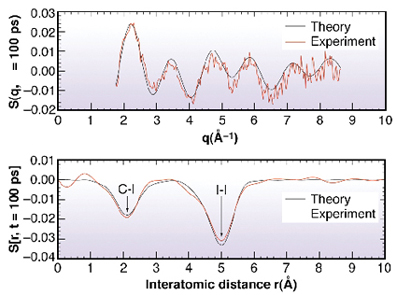 |
Fig. 23: The transition C2H4I4 |
The negative peak at 5.1 Å coincides with the I..I distance in C2H4I2. The measurements tell us that this correlation is reduced, i.e. at least one iodine atom has left the molecule after 100 ps. The second negative peak at 2.1 Å comes from a change in the C-I correlation. This feature is consistent with the formation of the bridged structure. The measurements at later times show, rather dramatically, that the liberated iodine atoms "swim" around the C2H4I radical and strips off the second iodine to form C2H4 + I2. This diffusive process takes about 10 ns. Note finally that the radial curves DS(r, t) provide a 1-dimensional moving image of a chemical reaction in solution: it is probably the closest we get to film chemical reactions in disordered media with X-rays.
References
[1] A. Plech, M. Wulff, S. Bratos, F. Mirloup, R. Vuilleumier, F. Schotte and P. A. Anfinrud, Phys. Rev. Lett., 92, 12, 125505-1 (2004).
[2] S. Bratos, F. Mirloup, R. Vuilleumier, M. Wulff and A. Plech, Chem. Phys. 304, 245251 (2004).
Principal Publication and Authors
H. Ihee (a), M. Lorenc (b), T.K. Kim (a), Q.Y. Kong (b), M. Cammarata (c), J.H. Lee (a) and M. Wulff (b), submitted for publication.
(a) Department of Chemistry and School of Molecular Science, Advanced Institute of Science and Technology (KAIST), 305-701, Daejeon (Republic of Korea)
(b) ESRF
(c) National Institute for the Physics of Matter and Department of Physical and Astronomical Sciences, Palermo (Italy)



