- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2003
- Macromolecular Crystallography
- Structural Genomics at the ESRF: studying Deinococcus radiodurans
Structural Genomics at the ESRF: studying Deinococcus radiodurans
The non-pathogenic red-pigmented Gram-positive bacterium Deinococcus radiodurans is extremely resistant to ionising radiation and other damaging agents such as ultraviolet (UV) radiation and desiccation. It is able to withstand up to 15,000 grays of ionising radiation and the mechanisms it uses to achieve this extraordinary extremophile capability have made this bacterium the subject of intense study. D. radiodurans was amongst the first organisms for which the complete genome sequence was made available and a thorough analysis of this genome has been carried out to try to elucidate the mechanisms by which this organism survives extreme radiation doses [1,2].
To complement such studies, the ESRF's Macromolecular Crystallography group has instigated a structural genomics initiative studying D. radiodurans. An initial round of protein targets have been selected for structural studies. Targets chosen included proteins involved in DNA-damage repair, desiccation resistance, and oxidative stress response. Additionally a number of hypothetical proteins with no known homologues in other organisms were also selected as targets.
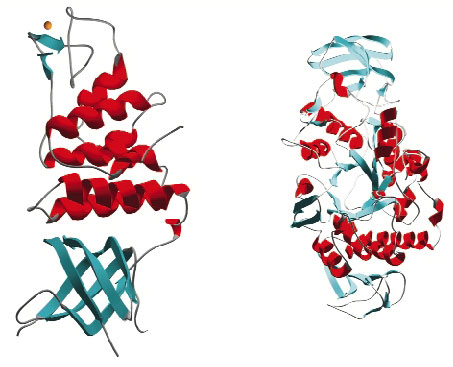
Once targets from the D. radiodurans R1 strain were chosen, cloning and small-scale protein expression testing was performed in collaboration with ProteinXpert (Grenoble). Using these results as a springboard we have thus far expressed 21 proteins on a scale necessary for crystallisation studies and successfully purified 18 of these. Crystallisation trials have produced diffraction quality crystals for 10 targets and of these 6 have proven amenable to structure solution (Figure 30). The structures were solved from data collected on the ESRF's macromolecular crystallography beamlines using single wavelength anomalous diffraction (SAD), multiple isomorphous replacement (MIR) and molecular replacement methods. At the time of writing a further seven targets are in crystallisation trials and native data have been collected from crystals of one target. The remaining projects are now the subject of further screening for large-scale expression and purification.
 |
|
Fig. 30: Examples of protein structures solved from Deinococcus radiodurans. |
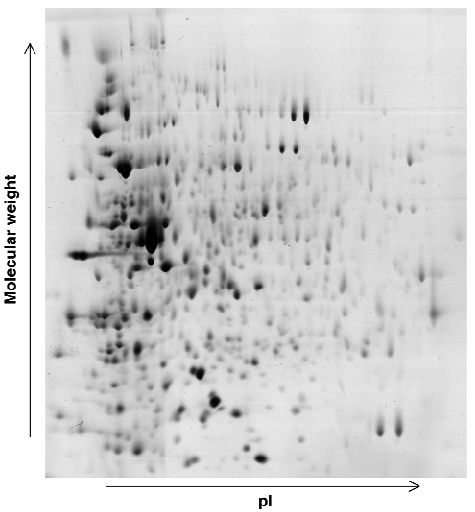
In conjunction with the work briefly described above, a study is under way using beamline ID17 to investigate the effects of radiation dosage on the expression levels of proteins within the cells of D. radiodurans. 2D-gel electrophoresis (Figure 31) is being used to identify changes in protein expression levels after exposure of D. radiodurans to large doses of radiation and during the recovery stages of the organism. Any proteins identified in these studies as contributing to the extremophile nature of D. radiodurans will then be investigated by a combination of molecular and structural biology.
 |
|
Fig. 31: Example of a 2D gel: the D. radiodurans proteome 30 minutes after irradiation. |
Reference
[1] White et al., Science, 286, 1571-7 (1999).
[2] Madarova et al., Microbiol. Mol. Biol. Rev. 65, 44-79 (2001)
Authors
D. Hall, I. Leiros, H-K. Leiros, E. Micossi, E. Gordon, S. Macedo, U. Kapp, C. Jamin, J. Timmins, G. Leonard, S. McSweeney.
ESRF



