- Home
- News
- Spotlight on Science
- Direct evidence...
Direct evidence of small airway closure in acute respiratory distress syndrome
16-09-2019
Airway closure is thought to play an important role in acute respiratory distress syndrome (ARDS). Airway closure has been imaged for the first time in an ARDS model by synchrotron phase contrast imaging providing direct evidence of this phenomenon.
ARDS is an acute inflammatory lung condition associated with high permeability oedema, surfactant dysfunction and widespread collapse of pulmonary alveoli, called atelectasis, which leads to decreased lung compliance and volume [1]. Clinicians have long suspected that the collapsibility of small airways is increased in this clinical syndrome, causing atelectasis [2,3]. While patients invariably require mechanical ventilation to survive, this life support measure can worsen lung injury due to exaggerated stress and strain applied to the tissue, which is magnified by mechanical inhomogeneity of lung tissue and atelectasis. Efforts to develop ventilation strategies that protect the lung, critically depend on our understanding of the mechanical behaviour of lung tissue and airways at the microscale. However, traditional computed tomography studies have not been able to clearly identify airway closure as a cause of atelectasis, due to their limited spatial resolution. To better identify the mechanisms involved in airway closure, it is necessary to use approaches that allow the study of individual airways. Here, the same individual small airways in intact lungs of anesthetised and mechanically ventilated rabbits with ARDS was studied using high resolution synchrotron phase-contrast computed tomography at beamline ID17.
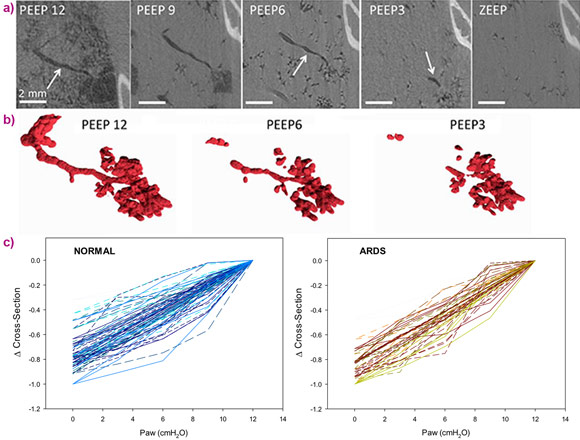
Propagation-based phase-contrast computed tomography, CT imaging, was performed with a 47.5 μm3 voxel size, at positive end-expiratory pressures (PEEP) of 12, 9, 6, 3 and 0 cm H2O. The imaging sequence was repeated after lung injury induced by surfactant depletion through whole lung lavage and high-tidal volume mechanical ventilation in anesthetised rabbits. Cross-sections of the same individual airways were measured (Figure 1). Airway collapsibility increased in the injured lung with a significantly faster drop in airway cross-section as airway pressure decreased in small airways ranging between 210 – 1690 µm in radius at 12 cmH2O.
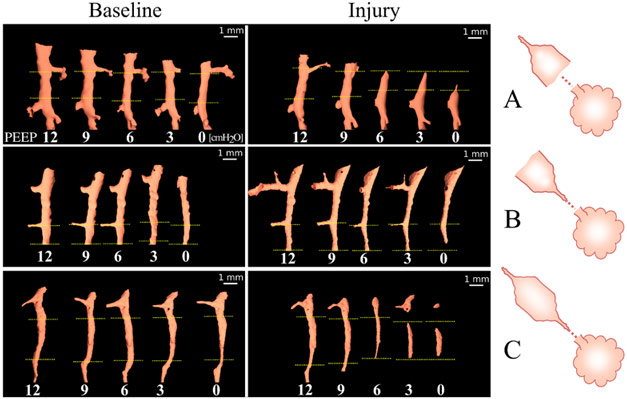
The main mechanism of airway closure in initially patent airways in ARDS was identified as “compliant collapse”. An example is shown in Figure 1a. Theoretical studies suggest that this mechanism involves an instability in the thickness of the fluid lining layer of the airway wall. Where this fluid layer thickens, the compressive forces that are generated cause a buckling of the airway wall that can lead to the collapse of the airway lumen along a certain length. Moreover, the analysis of airway cross-sections vs. length revealed that closure can occur at more than one site, sometimes leading to gas trapping and distension of the lumen (Figure 2).
Evidence of compliant collapse of airways in ARDS is indicated by the involvement of fluid layer movements at the microscopic level. This suggests that airway closure and reopening is not merely dependent on critical opening and closing pressures but is also a highly dynamic, time-dependent phenomenon [4]. This may have implications for patients with ARDS under mechanical ventilation. Strategies aiming at reducing the time during which respiratory pressure is lowered during the breathing cycle may help prevent airway closure.
This study also underscores the need for further development of fast, high-resolution imaging techniques to dynamically image lung tissue mechanics at the microscale [5], ideally within the time span of a breath. The crucial link between microscopic lung tissue strain, and initiation and maintenance of inflammation, which leads to high mortality levels in ARDS patients, is still to be investigated, and dynamic X-ray microscopy with synchrotron sources promises to pave the way.
Principal publication and authors
Individual airway closure characterized In vivo by phase-contrast CT imaging in injured rabbit lung, L. Broche (a,b), P. Pisa (c), L. Porra (d,e), L. Degrugilliers (f), A. Bravin (b), M. Pellegrini (a), J.B. Borges (a), G. Perchiazzi (a), A. Larsson (a), G. Hedenstierna (a), S. Bayat (g,h). Crit Care Med. 47, e774-e781 (2019). doi: 10.1097/CCM.0000000000003838.
(a) Hedenstierna Laboratory, Department of Medical Sciences, Uppsala University (Sweden)
(b) ESRF
(c) University of Picardie Jules Verne Medical Faculty, Amiens (France)
(d) Department of Physics, University of Helsinki (Finland)
(e) Helsinki University Central Hospital Medical Imaging Center, Helsinki (Finland)
(f) Department of Pediatric Intensive Care, Amiens University Hospital (France)
(g) University of Grenoble Alpes & Inserm UA7 STROBE Laboratory (France)
(h) Department of Pulmonology and Physiology, Grenoble University Hospital (France)
References
[1] Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. M.A. Matthay, & G.A. Zimmerman, Am J Respir Cell Mol Biol 33, 319-327 (2005); doi: 10.1165/rcmb.F305.
[2] Airway closure and closing pressure during mechanical ventilation, G. Hedenstierna & G.S. McCarthy, Acta Anaesthesiol Scand 24, 299-304 (1980).
[3] (Airway) Closure, at Last!, J.E. Baumgardner, Crit Care Med 47, 1281-1282 (2019); doi: 10.1097/ccm.0000000000003883.
[4] Dynamic Mechanical Interactions Between Neighboring Airspaces Determine Cyclic Opening and Closure in Injured Lung, L. Broche et al., Crit Care Med 45, 687-694 (2017); doi: 10.1097/CCM.0000000000002234.
[5] Mapping cardiac-induced lung motion using high-resolution time-resolved phase-contrast synchrotron computed tomography, L. Fardin et al., European Respiratory Journal 52, PA851 (2018; doi: 10.1183/13993003.congress-2018.PA851.
Top image: 3D-rendered segmentation of pulmonary blood vessels (red) and airways (blue) based on phase-contrast synchrotron radiation tomographic images. Image credit: S. Bayat.