- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2017
- Matter at extremes
- XAS reveals structure-activity relationships for the methane-to-methanol conversion of Cu-zeolites
XAS reveals structure-activity relationships for the methane-to-methanol conversion of Cu-zeolites
Methane-to-methanol (MTM) conversion in mild conditions represents a 'dream reaction', with an enormous impact on the energy sector and the chemical industry. Combining activity measurements with in situ/operando XAS, structure-activity relationships were established for the MTM process over Cu-SSZ-13 zeolites, shedding light on the nature of Cu-active sites.
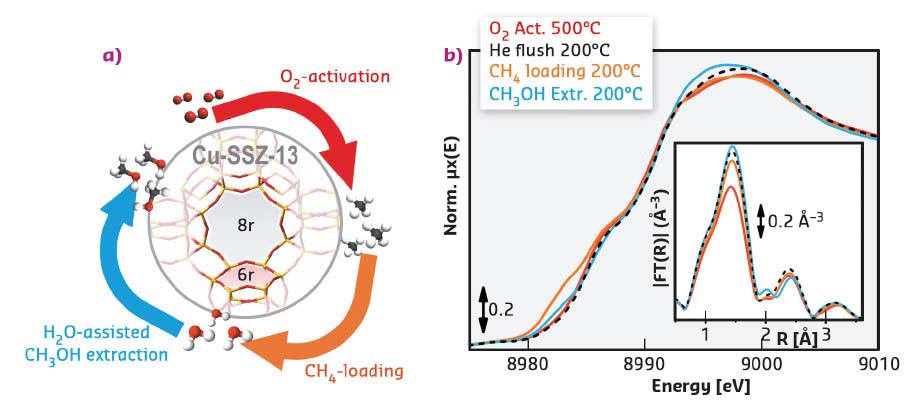
A less energy-intensive process allowing methane-to-methanol (MTM) conversion would represent a major breakthrough for chemical industry, with profound energetic and environmental implications. Although nature does the work masterfully with Cu enzymes [1], an industrially viable process for this conversion remains an ongoing challenge. In recent years, Cu-exchanged zeolites have been shown to possess Cu-active sites able to cleave the C−H bond of methane at temperatures ≤ 200°C, enabling its stoichiometric transformation into methanol [2]. The conversion is obtained through a stepwise process (Figure 41a), starting with a high-temperature activation in O2 to generate the active sites. Afterwards, methane is loaded over the material at 200°C and methanol is subsequently extracted by passing steam through the reactor hydrolysing the stabilised methyl intermediates.
 |
|
Fig. 41: a) The stepwise process for the MTM conversion over Cu-SSZ-13 zeolite, characterised by double 6-member (6r) and 8-member (8r) rings. b) Operando XANES and FT-EXAFS spectra collected at the BM26A beamline after each process step on a highly productive Cu-SSZ-13 sample. |
Laboratory activity measurements were combined with in situ/operando X-ray absorption spectroscopy (XAS) at beamlines BM23 and BM26A to explore the MTM conversion over Cu-SSZ-13 for a wide range of materials and reaction conditions, aiming at establishing structure-activity relationships. The oxidation state and average coordination of the Cu ions in the material were tracked by operando XAS during each step of the process (Figure 41b). XAS reveals tri-coordinated framework-interacting CuII centres as the dominant species in the O2-activated state at 500°C, while cooling to 200°C promotes an increase in the average first-shell Cu coordination number. During methane loading, XANES linear combination fit indicates the CuII to CuI reduction in 27% of the Cu sites. About half of these CuI sites (13% total Cu) undergoes re-oxidation during H2O-assisted methanol extraction, together with the formation of 27% of mobile CuII aquo complexes.
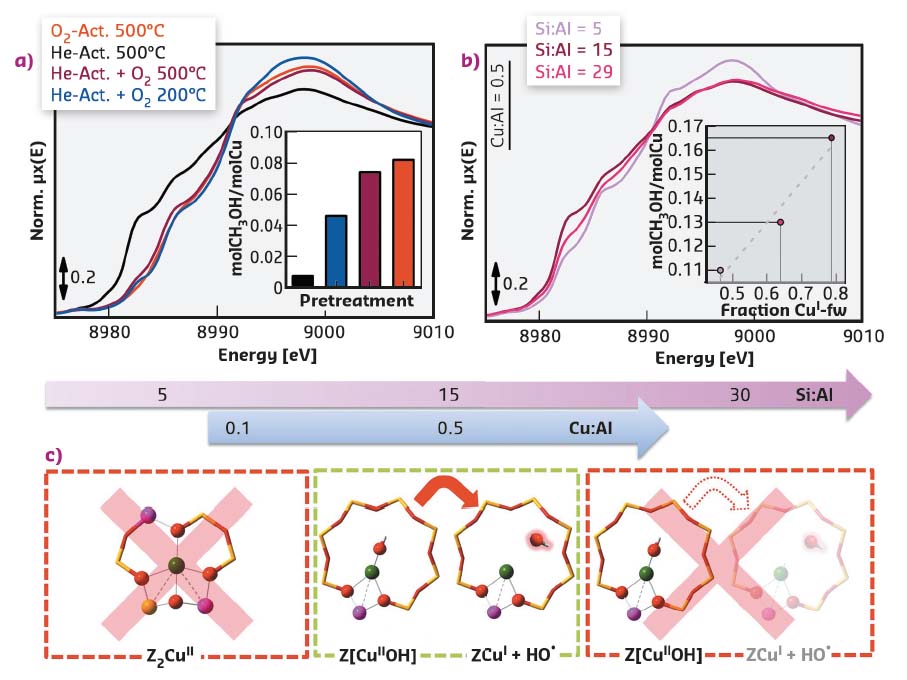
To obtain deeper insights in the nature of the active sites, the impact of different pre-treatments was explored by coupling in situ XAS with activity measurements (Figure 42a). High-temperature reaction with O2 is evidenced as a key requirement to form the methane-converting active sites. A positive linear correlation was also confirmed between the methanol productivity and the reducibility of the Cu centres under high-temperature treatment in He (Figure 42b). Here, for an accurate determination of Cu-speciation, previous results from multivariate analysis of a large composition-dependent XANES dataset were employed, also collected on BM23 [3].
 |
|
Fig. 42: In situ XANES collected on the BM23 beamline for: a) Cu-SSZ-13 with Cu/Al = 0.5, Si/Al = 12 after different pre-treatments (inset: correspondent normalised CH3OH productivities); b) He-activated Cu-SSZ-13 with Cu/Al = 0.5 and Si/Al ratio of 5, 15 and 29 (inset: linear correlation between the normalised productivity and the fraction of CuI species in the He-activated state. c) Rationalisation of the effect of composition on the productivity for the MTM conversion over Cu-SSZ-13. |
As depicted in Figure 42c, high populations of bare CuII sites charge-balanced by two proximal Al, favoured at low Si/Al ratios, inhibit the performance of the material by being inactive for the conversion. [CuIIOH]+ complexes are instead identified as the precursors to the active sites, through their ability to self-reduce, which is optimal at intermediate Si/Al ratios. As also supported by FTIR and Raman spectroscopy, [CuIIOH]+ species progressively deplete during high-temperature oxidative treatment, evolving towards different mono- and multimeric O2-derived CuII moieties, among which the active sites should be researched. These results mark an important step towards an improved understanding of reactive routes in Cu-zeolites, that will lead to rationalised material synthesis and potentially to an industrially feasible MTM process.
Principal publication and authors
D. K. Pappas (a), E. Borfecchia (b, c), M. Dyballa (a), I. A. Pankin (c, d), K. A. Lomachenko (d, e), A. Martini (a), M. Signorile (a), S. Teketel (b), B. Arstad (f), G. Berlier (a), C. Lamberti (c, d), S. Bordiga (a, c), U. Olsbye (a), K. P. Lillerud (a), S. Svelle (a) and P. Beato (b), Methane to methanol: structure-activity relationships for Cu-CHA, J. Am. Chem. Soc. 139, 14961-14975 (2017); doi: 10.1021/jacs.7b06472.
(a) Center for Materials Science and Nanotechnology (SMN), Department of Chemistry, University of Oslo (Norway)
(b) Haldor Topsøe A/S, Kgs. Lyngby (Denmark)
(c) Department of Chemistry, NIS Centre and INSTM Reference Center, University of Turin (Italy)
(d) International Research Center “Smart Materials”, Southern Federal University, Rostov-on-Don (Russia).
(e) ESRF
(f) SINTEF Materials and Chemistry, Oslo (Norway)
References
[1] R. L. Lieberman, A. C. Rosenzweig, Nature 434, 177-182 (2005)
[2] P. Tomkins et al., Angew. Chem. Int. Ed. 55, 5467-5471 (2016)
[3] A. Martini et al., Chem. Sci. 8, 6836-6851 (2017)



