- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2013
- Structure of materials
- Pushing the limits: giant negative compressibility
Pushing the limits: giant negative compressibility
When squeezed uniformly, the overwhelming majority of materials shrink in all directions. We have discovered that the crystalline compound zinc dicyanoaurate, Zn[Au(CN)2]2, actually expands under such hydrostatic pressure in a range of directions, and does so at a rate that is many times greater even than the usual compressibility of conventional ceramics. This negative linear compressibility (NLC) response has potential applications in the development of next-generation actuators, pressure sensors, and even artificial muscles [1].
The intuition that materials should shrink under pressure is grounded (rightly) in the underlying thermodynamics: pressure causes the free energy of a system to increase unfavourably unless there is an accompanying reduction in volume. The particular ‘trick’ of NLC materials such as Zn[Au(CN)2]2 is to couple volume reduction to linear expansion in one or more directions. In other words, their structures become longer as they become more dense. The wine-rack and honeycomb topologies are two examples of network geometries that respond to pressure in this way [2]. Indeed, we identified Zn[Au(CN)2]2 as a NLC candidate because its hexagonal crystal structure is based on the quartz topology, itself a three-dimensional honeycomb network (Figure 134).
![crystal structure of Zn[Au(CN)2]2](/files/live/sites/www/files/UsersAndScience/Publications/Highlights/2013/fig_134_HL2013.jpg) |
|
Fig. 134: The crystal structure of Zn[Au(CN)2]2 is assembled from tetrahedral Zn ‘nodes’, connected via almost-linear dicyanoaurate ‘linkers’ to give a three-dimensional network with the quartz topology. Under increasing hydrostatic pressure, this network reduces its volume by compressing along the a and b directions and expanding along the c axis. Rapid geometric changes are accommodated by compression of spring-like chains of gold atoms connected via weak ‘aurophilic’ interactions. |
Variable-pressure powder X-ray diffraction (PXRD) measurements, carried out using BM01A, the Swiss-Norwegian beamline, enabled us to determine how the crystal structure of Zn[Au(CN)2]2 changes when exposed to pressures of up to 14 GPa. From the variation in lattice parameters, we determined the crystal compressibilities, and by refining structural models against the diffraction intensities we could also monitor the accompanying changes in network geometry.
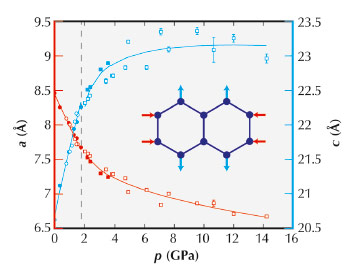
The compressibilities of conventional ceramics are usually around 5 TPa–1, meaning a decrease in length of 0.5% for each 1 GPa pressure interval. For Zn[Au(CN)2]2, we measured compressibilities of +52(6) and –42(5) TPa–1 along directions perpendicular and parallel to the [001] hexagonal crystal axis. These values become smaller at the very highest pressures (as one expects); nevertheless by the end of our experiment the sample had grown by roughly 10% along the NLC direction (Figure 135). The long-standing ‘record’ response amongst the dozen or so previously-known NLC materials was just –1.2 TPa–1 (for elemental selenium), making the ‘giant’ NLC effect of Zn[Au(CN)2]2 all the more remarkable.
 |
|
Fig. 135: Pressure-dependent variation in lattice parameters measured using PXRD. The NLC behaviour of Zn[Au(CN)2]2 is evident in the anomalous increase in the c lattice parameter with pressure. The vertical dashed line denotes a displacive phase transition to a related superstructure that also shows NLC. |
Our structural refinements revealed two key features responsible for such large compressibilities in this material. The first is that throughout the entire experiment there is remarkably little change to individual bond lengths and coordination geometries: the open framework structure, assembled from zinc ‘nodes’ and dicyanoaurate ‘linkers’ simply flexes in a way that would be expected to carry very little energy cost. The second feature is that the ‘shock’ of compressing this network so very rapidly in directions perpendicular to the [001] axis is accommodated by atomic-scale ‘springs’ assembled from highly polarisable gold atoms. So our study shows how a combination of structural engineering and supramolecular chemistry motifs might be coupled in order to design functional materials with counterintuitive mechanical responses.
Principal publication and authors
A.B. Cairns (a), J. Catafesta (b,c), C. Levelut (c) J. Rouquette (b), A. van der Lee (d), L. Peters (e), A.L. Thompson (a), V. Dmitriev (f), J. Haines (b) and A.L. Goodwin (a), Nature Materials 12, 212–216 (2013).
(a) Inorganic Chemistry Laboratory, Department of Chemistry, University of Oxford (UK)
(b) Institut Charles Gerhardt, Université Montpellier 2, Montpellier (France)
(c) Laboratoire Charles Coulomb, Université Montpellier 2, Montpellier (France)
(d) Institut Européen des Membranes, Université Montpellier 2, Montpellier (France)
(e) Institut für Kristallographie, RWTH Aachen, Aachen (Germany)
(f) Swiss-Norwegian beamline, ESRF
References
[1] R. Baughman, S. Stafstrom, C. Cui and S. Dantas, Science 279, 1522-1524 (1998).
[2] J. Grima, D. Attard, R. Caruana-Gauci and R. Gatt, Scr. Mater. 65, 565-568 (2011).



