- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2005
- Materials Science
- Mixed Interfullerene Bonding Motifs in C60-based Polymers
Mixed Interfullerene Bonding Motifs in C60-based Polymers
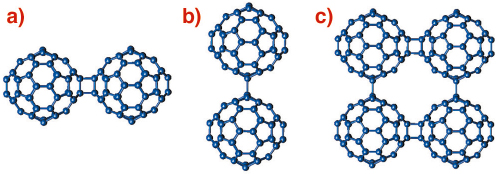
An unexpected discovery in fullerene chemistry has been the ease with which C60 units can covalently bond together to give rise to polymerised fullerene networks with a variety of structural architectures. Such fullerene-bridged arrays display varying dimensionality and intriguing electronic (metallic behaviour) and magnetic (ferromagnetism above room temperature) properties. The predominant interfullerene bonding structural motifs are either 4-membered carbon rings arising from [2+2] cycloaddition reactions (Figure 50a) or single C-C covalent bonds (Figure 50b) bridging together adjacent molecules and propagating in one (1D chains) or two (2D layers) dimensions [1].
 |
|
Fig. 50: Schematic drawing of the interfullerene C-C bridging structural motifs in polymeric fullerides. (a) [2+2] cycloaddition in AC60, (b) single C-C covalent bonds in Na2RbC60, and (c) mixed bonding in Li4C60. |
A prominent example of a fulleride polymer network is Li4C60whose structure has remained a subject of debate. When we probed its structural properties by high-resolution X-ray powder diffraction on beamline ID31, we found that Li4C60 adopts a layered polymeric structure. However, unexpectedly, and in contrast to all other known fullerene polymers, each C60 unit in Li4C60 bonds to its four nearest neighbours in the layers using both the [2+2] cycloaddition and the single C-C bridging motifs (Figure 50c), thereby giving rise to two types of differently-bonded chains running perpendicular to each other. The resulting unprecedented 2D fulleride network has neither been observed before experimentally nor been anticipated theoretically.
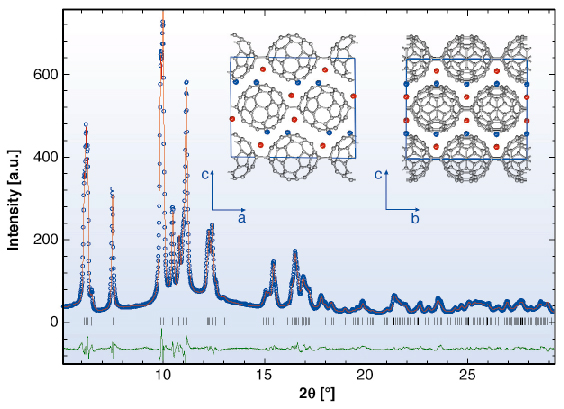
The X-ray powder diffraction profile of Li4C60 obtained at ambient temperature (Figure 51) revealed that its structure was body-centred monoclinic with lattice parameters, a = 9.3267(3) Å, b = 9.0499(3) Å, c = 15.03289(1) Å, and ß = 90.949(3)° (space group I2/m). A notable feature of this result is that Li4C60 is strongly anisotropic with the closest centre-to-centre contacts between the C60 units of ~ 9.33 Å, ~ 9.05 Å, and ~ 9.95 Å along the a and b axis and the body diagonal, respectively. While the latter contact is comparable to those encountered in monomeric fullerenes and fullerides (~ 10.0 Å), the one along b is reminiscent of that in monoclinic polymerised AC60 (~ 9.11 Å) in which there are two bridging C-C bonds between C60 – ions (Figure 50a). In addition, we note that short C-C contacts are also implied along the a axis, with the interfullerene distances comparing with those in fullerides with single C-C interfullerene connections (Figure 50b). The high-resolution synchrotron X-ray diffraction data allowed a detailed structural characterisation. Through a series of Rietveld refinements combined with Fourier analysis we were able to determine both the molecular bonding geometry and the precise location of the intercalated Li+ ions. Perspective views of the refined structure of Li4C60 highlighting the different bonding motifs on the ac and bc basal planes are shown in Figure 51 (inset).
 |
|
Fig. 51: Final observed (O) and calculated (–) synchrotron X-ray diffraction profile for Li4C60 at 295K ( |
The origin of the different structures adopted by Li4C60 and the related polymeric fullerides, Na2RbC60 (chains bridged by one C–C bond) and Na4C60 (layers bridged by four C–C bonds) is of considerable interest. The stability of polymeric fulleride structures is strongly associated with the charged state of the fulleride ions and the steric influence of the alkali ions. The small size of Li+ (0.60 Å) is of paramount importance in minimising steric crowding and allowing the incorporation of two Li+ in the space surrounding the pseudo-octahedral site. If Na2RbC60 or Na4C60 were to adopt structures similar to that of the Li4C60 polymer, severe steric hindrance would be encountered. The Rietveld refinements indicate a Li doping level of x = 3.49(7). Considering the usual behaviour of Li for partial electron donation to the fullerene units, a formal charge smaller than –4 is expected for the C60n- anions. This is again inconsistent with the adoption of the 2D Na4C60 structure, favoured for n ~ –4.
In this work, we have shown that the ground state of Li4C60 is a two-dimensional polymer with monoclinic crystal symmetry and an unprecedented architecture, combining both the [2+2] cycloaddition and the single C-C bridging motifs. This tightly-packed structure, whose salient features we have now confirmed by NMR and Raman spectroscopy [2], is the first example of a fullerene polymer with a mixed mode of interfullerene bridging and opens the way for the synthesis and study of new C60 –based polymers where different types of bonding geometries can coexist and interconvert by the application of external stimuli such as light irradiation or high pressure.
References
[1] S. Margadonna and K. Prassides, J. Solid State Chem. 168, 639 (2002).
[2] M. Riccò, T. Shiroka, M. Belli, D. Pontiroli, M. Pagliari, G.Ruani, D. Palles, S. Margadonna, M. Tomaselli, Phys. Rev. B 72, 155437 (2005).
Principal Publication and Authors
S. Margadonna (a), D. Pontiroli (b), M. Belli (b), T. Shiroka (b), M. Riccò (b), M. Brunelli (c), J. Am. Chem. Soc. 126, 15032-15033 (2004).
(a) School of Chemistry, University of Edinburgh (UK)
(b) Dipartimento di Fisica and CNISM, Università di Parma (Italy)
(c)ESRF



