- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2004
- Materials Science
- Reaction of Iron and Silica at Core-Mantle Boundary Conditions
Reaction of Iron and Silica at Core-Mantle Boundary Conditions
The boundary between the Earth's mantle and core (so-called D'' layer) is interesting because of the large contrast in properties across this region. The seismologically observed changes in density and sound wave velocities, for example, are significantly (2-3 times) greater than across the air-rock (or air-sea water) interface at the Earth's surface. Moreover, the difference in materials across the boundary, with predominantly crystalline rock above and liquid iron alloy below is among the most profound in the Earth. In this sense, the core-mantle boundary can be considered the primary "surface" of the planet, and is simply because of its remoteness that it has attracted less study than the top of the Earth's crust. There is a number of geophysical, geochemical, seismological arguments which link processes at D'' layer and at the surface of the Earth. For example, super-plumes originated from the core-mantle boundary (CMB) manifested themselves as hot-spot volcanoes on Hawaii.
The Moon and Sun cause the Earth's lunisolar precession and a so-called nutation of the Earth's rotation axis, i.e. small variations in the position of the Earth's rotation axis. It was recognised recently that the amplitude of the Earth's nutation is out-of-phase with tidal forcing produced by the Sun and Moon. A potential explanation for this observation could be the presence of a thin layer of a material with anomalously high electrical conductivity in the base of the Earth's mantle. What could be the nature of this layer?
To elucidate the composition and properties of the D'' layer at the core-mantle boundary, understanding of chemical reactions between liquid iron and the complex Mg-Fe-Si-Al-oxides of the Earth's lower mantle is required. Experimental problems arise due to extremely high pressures (over 1 400 000 atmospheres, or 140 GPa) and temperatures (over 3000°C), which characterise this region. We studied an interaction between iron and silica (SiO2) in electrically- and laser-heated diamond anvil cells and in a multianvil apparatus at pressures up to 140 GPa and temperatures over 3500°C, simulating conditions down to the CMB. At pressures below 40 GPa and high temperatures, iron and silica react forming iron oxide and iron-silicon alloy with up to 5 wt% Si (Figures 45 and 46).
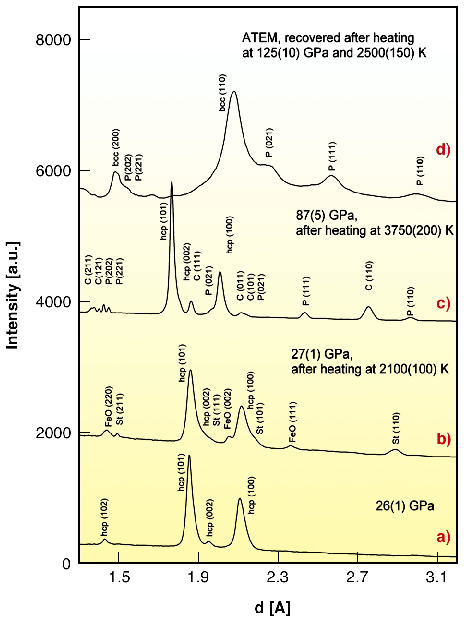 |
|
Fig. 45: Representative angle-dispersive X-ray (a-c) and electron (d) diffraction patterns obtained in experiments with iron and amorphous silica as starting materials. (a) At 26(1) GPa before heating of the sample only diffraction lines of |
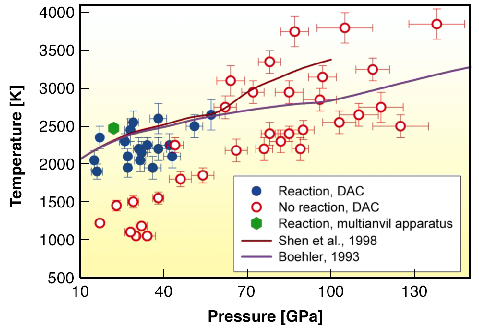 |
|
Fig. 46: Experimental results on the chemical interaction of iron and silica. Filled blue dots show P,T conditions at which the reaction between iron and silica was observed, and open red circles conditions at which the reaction does not occur. Green hexagon corresponds to observation of the reaction in multianvil experiments. Solid lines shows melting curve of iron according to Boehler (1993) (pink) and Shen et al. (1998) (dark-red). |
However, at pressures of 85-140 GPa, iron and SiO2 do not react and iron-silicon alloys dissociate into almost pure iron and the CsCl-structured (B2) FeSi compound (Figures 45 and 46). Experimental observations suggest that during formation and differentiation of proto-Earth, iron alloy, segregating in a deep magma ocean and thus containing several wt% Si would subsequently decompose into a mixture of Si-poor iron phase and silicon-rich B2 phase in the core. The metallic silicon-rich B2 phase, produced by this reaction, or produced at the CMB due to reaction between iron and silicate, is denser than the material of lower mantle and lighter than liquid iron in the Earth's outer core. So, iron silicide should accumulate at the CMB. It was found that high-pressure iron silicide phase B2 FeSi is an electrically conducting material. The presence of B2 FeSi at the base of Earth's lower mantle could explain the anomalously high electrical conductivity of this region and provide a key for understanding why the amplitude of the Earth's nutation is out-of-phase with tidal forcing.
Principal Publications and Authors
L. Dubrovinsky (a), N. Dubrovinskaia (a), F. Langenhorst (a), D. Dobson (a), D. Rubie (a), C. Geßmann (a), T. Le Bihan (b), W.A. Crichton (b), I. Abrikosov (c), B. Johansson (d), Nature, 422, 58-61, 2003; L. Dubrovinsky, N. Dubrovinskaia, F. Langenhorst, D. Dobson, D. Rubie, C. Geßmann, T. Le Bihan, W. A. Crichton, Phys. Earth Planet. Inter., 146, 243-247, 2004.
(a) Bayerisches Geoinstitut, Universität Bayreuth (Germany)
(b) ESRF
(c) Department of Physics and Measurement Technology, Linköpings University (Sweden)
(d) Condensed Matter Theory Group, Department of Physics, Uppsala University (Sweden)



