- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Structural biology
- Three-dimensional structures reveal molecular action of anti-smoking drug
Three-dimensional structures reveal molecular action of anti-smoking drug
Nicotine is the chemical substance in tobacco responsible for smoking addiction, which causes major public health risks. The effects of nicotine are mediated through specific nicotinic acetylcholine receptors (nAChRs) that function as heteropentameric ligand-gated ion channels composed of α4- and β2-subunits. These α4β2 nAChRs trigger downstream dopamine signalling in the mesolimbic system, which is an area in the brain that plays an important role in pleasure and reward sensation. Consequently, the α4β2 nAChR has become a key target for the development of therapeutic agents for smoking cessation. Recently, a partial agonist for the α4β2 nAChR, varenicline (Champix®), has been approved for clinical use as a smoking cessation aid (Figure 9a). This drug derives its therapeutic effect from two fundamental molecular actions. First, it binds to α4β2 nAChRs with higher affinity, but lower efficacy than nicotine. This effect is thought to antagonise the reward sensation of smoking. Second, varenicline activates and desensitises α4β2 nAChRs, thereby minimising withdrawal symptoms and increasing the success rate of smoking cessation attempts. Understanding the molecular mechanism of partial agonism of the nAChR has great potential for designing novel smoking cessation compounds but is currently hampered by the absence of a high-resolution structure of a eukaryote nicotinic receptor.
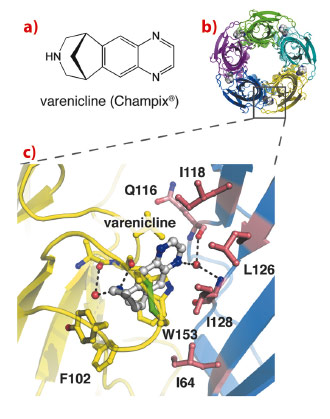 |
|
Fig. 9: X-ray crystal structure of Ct-AChBP in complex with varenicline. |
Current structural insight into ligand recognition by nAChRs derives from acetylcholine binding proteins (AChBPs), which are homologous to the extracellular ligand binding domain of nAChRs [1]. In this study, we demonstrate that a newly discovered AChBP from Capitella teleta, Ct-AChBP, binds partial agonists such as varenicline with high affinity, similar to α4β2 nAChRs. We determined X-ray crystal structures of Ct-AChBP in complex with varenicline (Figure 9b) or lobeline, which are both partial agonists at α4β2 nAChRs. These structures highlight the binding pocket architecture for molecular recognition of these ligands, revealing the contact residues that potentially mediate their molecular actions in α4β2 nAChRs (Figure 9c). The ligand binding site of Ct-AChBP is found at the interface between two subunits and ligands interact with amino acids from both the principal face (historically designated as ‘loops’ A, B, and C) and the complementary face (‘loops’ D, E, and F) of the binding pocket. On the principal face, varenicline interacts with aromatic amino acids (W153 in loop B and Y201 in loop C), which are highly conserved among different nAChR subtypes. These interactions include cation-Π interactions and multiple hydrogen bonds that converge on residues of loop B. On the complementary face of the binding site, varenicline forms mostly hydrophobic interactions with residues that are weakly conserved among different nAChR subtypes. In Ct-AChBP, these residues are I118, L126, and I128 in loop E and I64 in loop D. A water molecule forms hydrogen bonds between the varenicline pyrazino nitrogen and the backbone nitrogen and oxygen of I128 and Q116, respectively. Remarkably, this water molecule occupies a position that is almost identical to the water molecule observed in the complex between AChBP and nicotine [2].
Employing structure-based mutagenesis, we investigated the functional role of several homologous contact residues in human α4β2 nAChRs expressed in Xenopus oocytes. Using the two-electrode voltage-clamp technique, we measured ligand-activated currents of nAChRs and compared relative affinity and efficacy for varenicline and desensitisation by varenicline and the full agonist acetylcholine on wilde type and mutant receptors. We found that mutations at two positions, namely W57 and L121, have remarkable effects on channel function. First, we observed that the mutations abolish varenicline-mediated desensitisation of α4β2 nAChRs (Figure 10). This result suggests that the loop D residue W57 (I64 in Ct-AChBP) and loop E residue L121 (I128 in Ct-AChBP) form critical interactions with varenicline to mediate transitions to a densensitised state of the α4β2 nAChRs. Second, we observed that mutations at these positions eliminate the relative difference in efficacy of channel opening between varenicline and the full agonist acetylcholine. Together, these results demonstrate the predictive value of the X-ray crystal structure and highlight the key role of specific amino acid residues in mediating receptor desensitisation and channel opening with limited efficacy, which both are effects thought to facilitate smoking cessation.
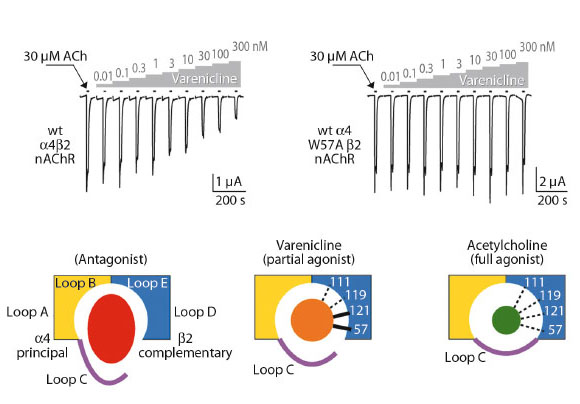 |
|
Fig. 10: Ligand-activated currents of nAChRs. |
In summary, our results help to understand the effects of smoking cessation drugs in a structural context and provide a novel entry into drug discovery programmes aimed at developing better smoking cessation aids.
Principal publication and authors
B. Billen (a), R. Spurny (a), M. Brams (a), R. van Elk (b), S. Valera-Kummer (c), J.L. Yakel (d), T. Voets (e), D. Bertrand (c) and A.B. Smit (b), C. Ulens (a), Proc Natl Acad Sci U S A 109, 9173-9178 (2012).
(a) Laboratory of Structural Neurobiology, KULeuven (Belgium)
(b) Department of Molecular & Cellular Neurobiology, VU University (The Netherlands)
(c) HiQScreen, Geneva (Switzerland)
(d) Laboratory of Neurobiology, NIEHS (USA)
(e) Laboratory of Ion Channel Research, KULeuven (Belgium)
References
[1] K. Brejc, W.J. van Dijk, R.V. Klaassen, M. Schuurmans, J. van Der Oost, A.B. Smit and T.K. Sixma, Nature. 411, 269-276 (2001).
[2] P.H. Celie, S.E. van Rossum-Fikkert, W.J. van Dijk, K. Brejc, A.B. Smit and T.K. Sixma, Neuron 41, 907-914 (2004).



