- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Structural biology
- The molybdenum storage protein combines protein and inorganic polyoxometalate cluster chemistry
The molybdenum storage protein combines protein and inorganic polyoxometalate cluster chemistry
Molybdenum plays an essential role in many vital biochemical processes, for which organisms have developed a complete Mo metabolism including uptake/transport, gene regulation, homeostasis and storage. Mo is used within complex cofactors (molybdopterin in diverse oxygen-transferring redox enzymes and an FeMo cofactor in nitrogenases) or simply as single molybdate ions bound to proteins. Recent studies on a Mo storage protein (MoSto) of the N2 fixing bacterium Azotobacter vinelandii revealed that Nature also uses Mo in the form of polyoxometalate clusters. Inorganic polyoxometalate chemistry is a long-standing research field. Polyoxometalate clusters are characterised by their huge variety culminating in “giant clusters” of up to 368 Mo atoms.
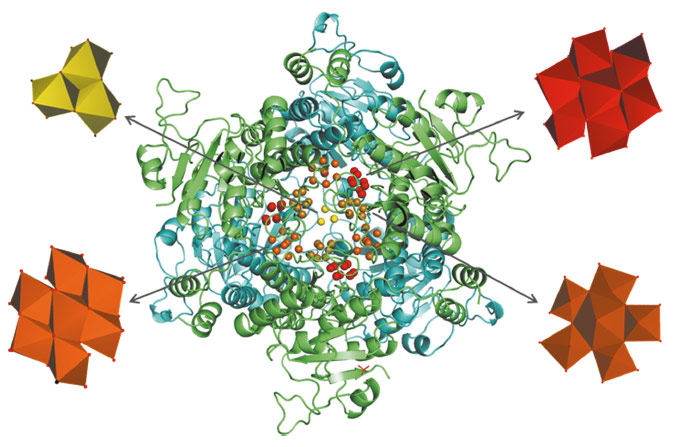
The MoSto protein in A. vinelandii is related to the Mo-dependent nitrogenase: it supplies the nitrogenase cofactor with Mo to ensure nitrogenase functionality under molybdenum-deficient conditions. Storage as polyoxomolybdate allows the organism to compactly deposit more than 100 Mo ions in a single MoSto molecule. Molybdate uptake is an ATP hydrolysis driven process; molybdate release is ATP-independent but pH-regulated, occurring stepwise above pH 6.8 [1]. X-ray structure analysis of the MoSto protein at 1.6 Å resolution revealed a cage-like (αβ)3 protein complex with a central cavity approximately 7250 Å3 in volume (Figure 6). Well-defined pockets on the surface of the cavity form the binding sites for various polyoxometalate clusters. To correctly assign the type of each cluster atom at the corresponding position we performed X-ray fluorescence scans (0.6-2.1 Å) and single-wavelength anomalous dispersion experiments (1.73 Å) on MoSto crystals at beamline ID29. Elements (such as magnesium and phosphorous) with absorption edges outside the wavelength spectra of the scans could be distinguished from Mo by their low anomalous dispersion signal. On this basis we detected about 100 Mo atoms in the cavity, 70 of them in defined polynuclear Mo-O aggregates. The ten polymolybdate clusters observed were subdivided into four distinct types: one Mo32, three Mo5-7 clusters and six structurally identical Mo8 clusters. Three of the Mo8 clusters are bound covalently, while the other three are bound non-covalently (see Figure 6). Interestingly, MoSto can also be loaded with polynuclear tungsten-oxygen clusters [2].
Since polyoxomolybdate clusters are not formed spontaneously at neutral or weakly acid pH values, molybdate polymerisation to larger polyoxometalate aggregates must occur inside the protein cavity. Two scenarios of polyoxometalate cluster synthesis can thus be distinguished: (a) the protein binding pocket acts as template, nucleation site and catalyst of the polymerisation reaction thus predetermining the type (size, shape) of the cluster and (b) small clusters are spontaneously and transiently formed under bulk conditions (perhaps promoted by special electrostatic conditions in the solvent-filled cavity) and selectively stabilised by the protein matrix in a subsequent process. In both cases the protein matrix protects/stabilises the metal clusters by binding them and preventing them from rapid degradation. The protein/template-induced and self-assembly contributions differ from cluster to cluster.
MoSto is unique in biology and extends the competences of proteins in general, by synthesising/binding/protecting polynuclear metal-oxide clusters of different types. It combines macromolecular biochemistry with supramolecular inorganic chemistry, both characterised by a tremendous structural diversity. This quality could eventually be exploited by using the protein cavity with its tailor-made pockets as a bioreactor for synthesising novel polyoxometalate clusters in a controllable and tunable way.
Principal publication and authors
B. Kowalewski (a), J. Poppe (b), U. Demmer (b), E. Warkentin (b), T. Dierks (a), U. Ermler (b) and K. Schneider (a), J. Am. Chem. Soc. 134, 9768-9774 (2012).
(a) Department of Biochemistry I, University of Bielefeld (Germany)
(b) Max-Planck-Institute for Biophysics, Frankfurt am Main (Germany)
References
[1] J. Schemberg, K. Schneider, D. Fenske and A. Müller, ChemBioChem 9, 595-602 (2008).
[2] J. Schemberg, K. Schneider, U. Demmer, Warkentin, A. Müller and U. Ermler, Angew. Chem. 119, 2460-2465 (2007).