- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2012
- Structural biology
- Bacterial cell division protein FtsA forms actin-like filaments
Bacterial cell division protein FtsA forms actin-like filaments
The divisome is the inner membrane protein complex performing bacterial cell division, leading to two daughter cells. It consists of more than a dozen proteins, with one component being FtsZ, the bacterial tubulin homologue forming the Z-ring [1,2]. In many bacteria, FtsZ is assisted by FtsA, which displays structural similarities to actin but shows an altered subdomain architecture when compared to actin, ParM or MreB [3]. FtsA contains an amphipathic helix for its attachment to the inner membrane and it has been shown that the two proteins, FtsA and FtsZ, interact directly [4]. Whether FtsA is able to polymerise, given its unusual actin-like fold, has been a long-standing question.
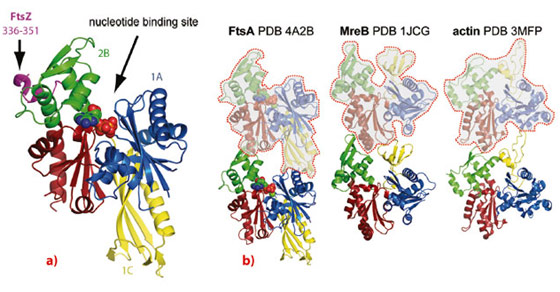
We employed a combination of biochemical and structural techniques, including X-ray crystallography at the ESRF. First, we set out to define the minimal portion of FtsZ that interacts with FtsA and reconstituted the FtsA:FtsZ:membrane system in vitro. By means of co-pelleting assays we demonstrated that the last 8-16 residues of Thermotoga maritima FtsZ (TmFtsZ) are necessary and sufficient to interact with T. maritima FtsA (TmFtsA) in vitro. We also confirmed that the C-terminal amphipathic helix of FtsA is required for membrane binding. We then co-crystallised full-length TmFtsA with the peptide corresponding to the last 16 residues of TmFtsZ. A high-resolution structure, solved from a dataset obtained at beamline ID29, revealed that the peptide bound within the 2B subdomain of TmFtsA between helices H6 and H8, adopting a predominantly alpha-helical conformation (Figure 21a). The protein was present as a translational dimer in the asymmetric unit and when co-crystallised with ATPγS, TmFtsA produced new crystals containing continuous protofilaments that closely resemble the dimer.
Importantly, these filaments are related in their architecture and subunit arrangement to all known actin-like protofilaments, including actin itself, ParM and MreB (Figure 21b). In order to investigate FtsA’s polymerising abilities biochemically, we took advantage of FtsA’s membrane affinity and utilised an electron microscopy monolayer assay. This revealed filaments several hundred nanometres in length, often present as doublets. Occasionally we could observe two-dimensional sheets. Computational Fourier transformation of the images of the sheets provided us with a longitudinal spacing of around 48 Å, which matches the spacing found in the crystals.
To investigate FtsA polymerisation in a broader range of bacterial species we turned to electron cryotomography and structured illumination fluorescence microscopy (SIM) and over-expressed FtsA from Thermotoga, Escherichia coli and Bacillus subtilis in E. coli cells. This confirmed our previous in vitro studies and we were able to visualise FtsA filaments in cells. Interestingly, full-length TmFtsA bound to the inner surface of the cytoplasmic membrane through its C-terminal amphipathic helix thereby distorting the membrane and forming lipid structures, coated with FtsA protein filaments. Finally, we investigated whether FtsA polymers are of biological significance in vivo. For this, we first rationally designed polymerisation-deficient mutants based on our high-resolution crystal structure of the polymer. Then we confirmed lack of polymerisation by means of SIM and performed a complementation assay in a B. subtilis FtsA temperature sensitive (ts) background, in which the ts-FtsA protein is unable to support cell division at elevated temperatures and this endogenous tsFtsA is complemented with a series of mutant versions of FtsA, polymerising and non-polymerising. We were able to observe that the polymerisation-deficient mutants did not rescue the wild-type phenotype and cells were elongated and filamentous. We therefore concluded that FtsA polymerisation has an important role in bacterial cell division.
In this work, we showed that FtsA appears to be a domain-swap of the canonical actin fold that is capable of forming protofilaments because the swapped 1C domain appears in the correct place in the protofilament, shifted by one subunit (Figure 21b).
Principal publication and authors
P. Szwedziak, Q. Wang, S.M. Freund and J. Löwe, EMBO J 31, 2249-60 (2012).
MRC Laboratory of Molecular Biology, Cambridge (UK)
References
[1] D.W. Adams and J. Errington, Nat Rev Microbiol 7, 642 (2009).
[2] J. Löwe and L.A. Amos, Nature 391, 203 (1998).
[3] F. van den Ent and J. Löwe, EMBO J 19, 5300 (2000).
[4] S. Pichoff and J. Lutkenhaus, Mol Microbiol 64, 1129 (2007).