- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2011
- Enabling technologies
- Synchrotron radiation microprobing of biological matter manipulated by optical tweezers
Synchrotron radiation microprobing of biological matter manipulated by optical tweezers
Sample manipulation in synchrotron radiation experiments is commonly performed through mechanical contact which may induce mechanical deformations in particular for biological and soft matter materials in aqueous environments. An alternative to mechanical devices is optical tweezers which are based on the trapping capability of focused laser beams with contact forces in the femto- to nano-Newton range [1]. Optical tweezers can be used for manipulation of single particles, assembly and rotation. They also allow the generation of multiple traps, thus providing the possibility of distributing the radiation dose on several micro-objects which is of particular interest for experiments with intense synchrotron radiation micro- and nano-beams. The ability to rotate single particles allows, in principle, the development of an optical goniometer for diffraction, tomography and imaging experiments. Investigations of single fragile objects manipulated by optical forces with high brilliance X-ray beams may enable new research fields such as serial protein crystallography at storage rings and even at XFEL sources.
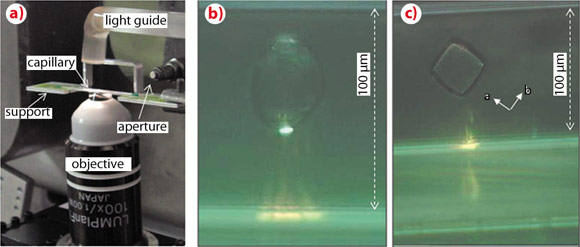
We have developed a compact and portable optical tweezers setup for synchrotron radiation applications at the ID13 beamline [2]. Objects of several micrometres up to few tenths of a micrometre in size can be trapped for extended periods of time. A laser beam of λ = 1070 nm is focused by a high numerical aperture immersion objective into a glass capillary filled with the particles suspended in an aqueous environment (Figure 147a). The trapped objects studied were typically starch granules, and insulin crystals (Figures 147b,c). The selection and positioning of single objects out of a batch of many can be performed currently in a semi-automatic manner by software routines.
 |
|
Fig. 147: a) Optical tweezers sample environment; the laser beam is focused by an immersion objective into the capillary. b,c) Optically trapped starch granule and insulin crystal viewed along the beam direction. The trace of the laser beam is visible through the capillary. The crystallographic orientation of the cubic insulin unit cell is indicated. |
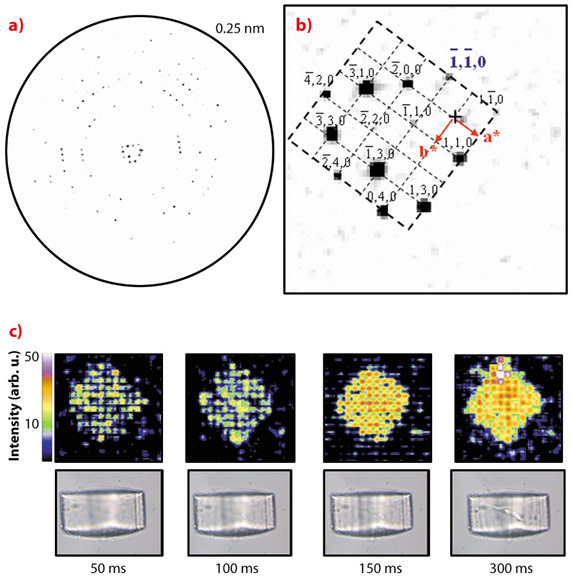
We have demonstrated the feasibility of microdiffraction experiments on optically trapped starch granules and protein crystals which were repeatedly raster-scanned at 50 ms exposure/raster-point with an about 1 micrometre beam of wavelength λ ~0.09 nm at around 13 keV [2]. Indeed, for the cubic insulin crystal shown in Figure 147c, order along the densely packed [110] direction is maintained until complete loss of long-range order. The average of all patterns collected during the first raster-scan with 50 ms/point is shown in Figure 148a. The inner part of the reciprocal lattice has been indexed for the cubic I213 space group (Figure 148b). The evolution of the crystal diffracting properties with increasing dose is revealed during the successive raster-scans in a composite diffraction image with the intensities of the pixels reflecting the local -1-10 reflection intensity (Figure 148c). We observe that the general shape of the crystal is maintained up to about 300 ms exposure when plotting successive composite diffraction images. During this period, the resolution limit of the crystal rapidly shrinks until only the inner hk0 reflections remain visible (not shown). The strong intensity fluctuations visible in the 50/100 ms composite diffraction images disappear with increasing radiation damage suggesting the evolution of a more homogeneous domain size.
 |
|
Fig. 148: a) Averaged diffraction pattern of the insulin crystal raster-scan at 50 ms exposure/point. b) The inner part of the hko reciprocal lattice. c) Upper row: evolution of the insulin composite diffraction images with increasing exposure time for the -1-10-reflection intensity. The times indicated correspond to the overall exposure time per point for each raster-scan. Lower row: Optical microscopy images recorded normal to the X-ray beam direction via the immersion objective. The radiation damage induced by the microbeam is revealed by stripes. |
Our experiments demonstrate the potential of optical tweezers for manipulating objects requiring ultra-low contact forces. We think that the possibilities for room temperature protein crystallography experiments in an aqueous environment are particularly interesting in view of crystals which are difficult to cryofreeze. As the setup is compact and flexible, further customisation is possible for ad-hoc requirements of specific applications under X-rays such as particle shaping and assembly as well as particle fractionation.
Principal publication and authors
S.C. Santucci (a,b), D. Cojoc (b), H. Amenitsch (c), B. Marmiroli (c), B. Sartori (c), M. Burghammer (a), S. Schoeder (a,d), E. Di Cola (a), M. Reynolds (a) and C. Riekel (a), Analytical Chemistry 83, 4863-4870 (2011).
(a) ESRF
(b) CNR-IOM Laboratorio TASC, Trieste (Italy)
(c) IBS, Graz (Austria)
(d) Soleil Synchrotron (France)
References
[1] A. Ashkin, Optical trapping and manipulation of neutral particles using lasers. World Scientific Publishing Co. Pte. Ltd. (2006).
[2] S.C. Santucci et al., Analytical Chemistry 83, 4863-4870 (2011).



