- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2010
- Soft condensed matter
- Controlling the growth and shape of chiral supramolecular polymers in water
Controlling the growth and shape of chiral supramolecular polymers in water
Until recently, self-assembly in dilute aqueous environments has predominantly dealt with linear amphiphiles that form closed structures, such as spherical or cylindrical micelles, and vesicles. Morphological control in objects of defined size or shape is increasingly well understood. Surprisingly, however, the generality of these concepts has not been translocated into another area of increasing interest, namely, the self-assembly of one-dimensional arrays. The development of discotic monomers has proven to be a valuable route to allow the synthesis of rod-like supramolecular polymers that have potential applications in functional soft matter including electronics, sensing and regenerative medicine. Considering the enormous interest in such systems, it is surprising that efforts to control the size and shape of nano- and micrometre size one-dimensional objects are rare [1]. To manipulate the growth of aqueous one-dimensional supramolecular polymers, we have utilised electrostatic repulsive contributions in analogy to surfactant type self-assembly. However, rather than simply relying on solvophobic effects based on the lipophilic segments of the amphiphile, we have used noncovalent polymerisable moieties to craft building blocks that are designed to code for ordered supramolecular architectures.
 |
|
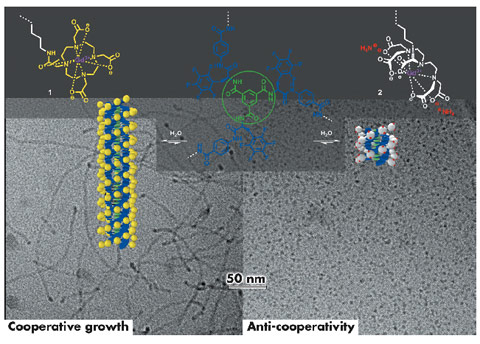
Fig. 68: Schematic representation of the self-assembly of the discotic amphiphiles 1 and 2. |
The molecular design of our self-assembling unit is based on the well-studied C3-symmetrical benzene-1,3,5-tricarboxamide (BTA) core (Figure 68, depicted in green) that directs the self-assembly into triple hydrogen bonded helices. This moiety was extended with a fluorinated L-phenylalanine and an aminobenzoate spacer (Figure 68, depicted in blue), creating a hydrophobic pocket in the core of the discotic to shield the triple hydrogen-bonding motif. By increasing the ionic character of the peripheral Gd(III) complexes in the charge neutral discotic 1 to the negatively charged discotic 2, we aimed at introducing frustration in the one-dimensional growth of the stacks. Cryo-TEM imaging at the Eindhoven University of Technology and small angle X-ray scattering (SAXS) experiments on beamline BM26B revealed that it is possible to switch from elongated, rod-like assemblies to small and discrete objects (Figures 68 and 69), by balancing attractive non-covalent forces within the hydrophobic core of the polymerising building blocks with electrostatic repulsive interactions on the hydrophilic rim.
 |
|
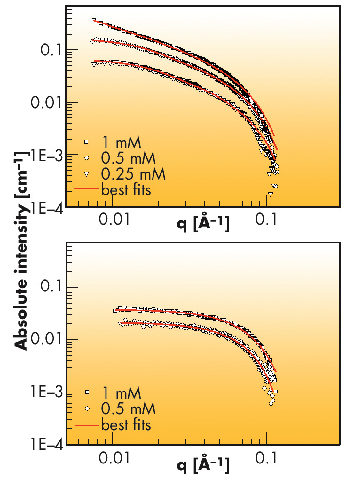
Fig. 69: SAXS profiles of the self-assembled discotics 1 (upper graph) and 2 (lower graph) at different concentrations. |
Furthermore, the concentration dependent aggregation process of rod-like stacks could be studied as suggested by SAXS profiles: at 0.25 mM of discotic 1, rods of 23 nm in length and 6.2 nm in diameter are formed. At 0.5 mM the rod length is 27 nm and at 1 mM the length increases well above 75 nm. The order in the self-assembled objects and their growth mechanism were also characterised using circular dichroism, UV/Vis and 1H-NMR spectroscopy. In line with our continuous efforts in elucidating the mechanisms of supramolecular polymerisations [2], we have focussed on correlating the morphological properties of the materials with the appropriate mechanistic details of the self-assembly pathways: cooperative growth of monomer 1 leads to very high molecular weight supramolecular polymers, whereas frustrated growth of discotic 2 and the resulting anticooperativity, results in the formation of small and discrete objects, without compromising their thermodynamic stability. The latter are very promising building blocks for the development of supramolecular magnetic resonance imaging (MRI) contrast agents.
This is a unique example for directional self-assembly in water whereby the supramolecular polymer shape and size can be dictated by Coulombic interactions. In analogy to many systems found in nature, mechanistic details of the self-assembly process emphasise the importance of cooperativity as a key feature that dictates the physical properties of the supramolecular polymers.
Principal publication and authors
P. Besenius (a), G. Portale (b), P.H.H. Bomans (c), H.M. Janssen (d), A.R.A. Palmans (a) and E.W. Meijer (a), Proc. Natl. Acad. Sci. U.S.A. 107, 17888-17893 (2010).
(a) ICMS and MST, Eindhoven University of Technology (The Netherlands)
(b) DUBBLE, ESRF
(c) SMG, Eindhoven University of Technology (The Netherlands)
(d) SyMO-Chem (The Netherlands)
References
[1] L.C. Palmer, Y.S. Velichko, M. Olvera de la Cruz and S.I. Stupp, Phil. Trans. R. Soc. A 365, 1417-1433 (2007).
[2] T.F.A. de Greef, M.M.J. Smulders, M. Wolffs, A.P.H.J. Schenning, R.P. Sijbesma and E.W. Meijer, Chem. Rev. 109 5687-5754 (2009).



