- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2009
- X-ray imaging
- The metallobiology of neuromelanin pigment in the human brain studied by synchrotron X-ray microspectroscopy
The metallobiology of neuromelanin pigment in the human brain studied by synchrotron X-ray microspectroscopy
Neuromelanin is a dark coloured granular pigment which forms within dopamine and noradrenaline-containing neurons of the substantia nigra (SN) pars compacta and the locus coeruleus in the human brain (Figure 131a). Little is known about this uniquely human pigment; its structure, biosynthesis pathway and physiological functions are not yet completely understood. We have recently demonstrated that neuromelanin production in human dopamine neurons occurs in three phases; an initiation phase occurring around the age of three, growth in pigment volume during adolescence and maturation and darkening of the pigment in adulthood [1]. One hypothesised role for neuromelanin is metal homeostasis; metal-handling systems are suggested to be dysfunctional in a number of neurodegenerative diseases. Interestingly, neuromelanin-containing neurons degenerate dramatically in the common neurodegenerative disorder Parkinson’s disease. Thus, it is important to examine the cellular metallobiology of neuromelanin in the healthy brain and its possible role in the pathogenesis of Parkinson’s disease.
The isolation of neuromelanin from the human SN is an intricate procedure that may alter the physicochemical integrity of neuromelanin granules and requires pooling multiple brain samples to obtain sufficient yield for standard biochemical and biophysical techniques. An alternative is a detailed intracellular analysis of neuromelanin. To meet these challenges, we employed a synchrotron microprobe and the recently developed nanoprobe (ID22NI) to characterise the microchemical environment of neuromelanin in tissue sections from both young and mature human brains in situ and for the first time (Figure 131b).
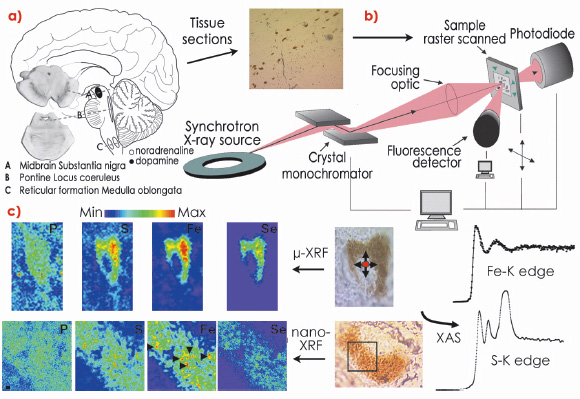 |
|
Fig. 131: a) The three main regions containing neuromelanin-producing cells in the human brain: A, substantia nigra of the midbrain; B, locus coeroleus within the pons; C, ventrolateral reticular formation in the medulla oblongata. b) Scheme showing the different steps involved in the synchrotron X-ray microspectroscopic analysis of pigmented neurons of the substantia nigra. The 8 µm thick brain tissue sections are mounted on a thin polymer film. c) Iron (Fe) and sulphur (S) intracellular speciation (X-ray absorption spectroscopy, XAS) were performed at ID22 and ID21 respectively. Using X-ray fluorescence (XRF), neuromelanin-containing neurons were raster scanned to generate a pixel-by-pixel spectral image providing topographical elemental maps. Arrow heads show iron-rich microdomains within neuromelanin aggregates. |
Our quantitative data demonstrated a marked increase in many neuromelanin-associated elements during the intense period of physical and brain development during childhood and adolescence, with smaller but progressive increases with aging. Many of the elements present in the pigment are crucial for normal brain function, particularly in the SN. Neuromelanin is the major Fe storage site in SN neurons in healthy individuals [1] and, using whole brain analysis, a linear increase in Fe concentration with age in the SN has been reported. Our results confirmed this trend and extend it to the intracellular level for the different neuromelanin maturation stages. Furthermore, Fe speciation obtained via X-ray absorption microspectroscopic analysis showed that Fe within neuromelanin exists in a configuration similar to that in ferritin (a Fe-binding protein present in glial cells in the brain) and that Fe is stored in a Fe3+ active form at all neuromelanin developmental stages. Significantly, the nanoprobe enabled us to visualise the localisation of essential elements associated with neuromelanin at a resolution (80 nm) not previously achievable and demonstrated a heterogeneous distribution of various elements within the pigment itself (Figure 131c). Fe-rich microdomains exhibiting irregular shapes were of micrometre or submicrometre size and were most apparent in the elderly with larger numbers of Fe-rich areas rarely observed in the less mature pigment. Variability in the polymer’s metal binding domains suggests that these sites may serve different functions, with labile sites available for cellular reactions and denser sub-micrometre aggregates that irreversibly scavenge potentially neurotoxic species.
We also found a marked increase in Se concentrations in neuromelanin with age, accompanied by a net increase of Mn in the elderly brain. While the chemical form of selenium in neuromelanin could not be investigated in the current study, this finding suggests a stimulation of cellular defence mechanisms mediated by selenoproteins during aging.
Finally, S speciation studies at the submicrometre level revealed the presence of reduced sulphur compounds and various forms of oxidised sulphur compounds in neuromelanin. Furthermore, a significant increase in sulfonate in neuromelanin in the mature brain suggests in vivo metabolism of the pigment occurring via an as yet unidentified pathway.
Such micro- and nano- analytical methods with high sensitivity, multiple-element and speciation capabilities, requiring only a single tissue section, will open exciting new avenues for the study of intracellular biotransformation pathways, particularly the role of trace metals in the brain.
Reference
[1] K.L. Double, V.N. Dedov, H. Fedorow, E. Kettle, G.M. Halliday, B. Garner and U.T. Brunk, Cell Mol Life Sci. 65, 1669-1682 (2008).
Principal publication and authors
S. Bohic (a,b), K. Murphy (c), W. Paulus (d), P. Cloetens (b), M. Salomé (b), J. Susini (b) and K. Double (c), Anal Chem. 80, 9557-9566 (2008).
(a) INSERM U-836 (Team 6, Rayonnement Synchrotron et Recherche Médicale), Grenoble Institut des Neurosciences (France)
(b) ESRF
(c) Prince of Wales Medical Research Institute and the University of New South Wales (Australia)
(d) Institute of Neuropathology, University Hospital, Munster (Germany)



