- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- X-ray Absorption and Magnetic Scattering
- Structural studies of Sr(FexTi1-x)O3-δ solid solutions by XAS and Raman spectroscopy
Structural studies of Sr(FexTi1-x)O3-δ solid solutions by XAS and Raman spectroscopy
The perovskite solid solution series Sr(FexTi1–x)O3–![]() , 0
, 0 ![]() x
x ![]() 1, is an interesting system spanning the range from slightly iron-doped SrTiO3 as a model representative of acceptor-doped large band gap electroceramics, to iron-rich Sr(FexTi1–x)O3–
1, is an interesting system spanning the range from slightly iron-doped SrTiO3 as a model representative of acceptor-doped large band gap electroceramics, to iron-rich Sr(FexTi1–x)O3–![]() materials, which are good electronic and ionic conductors. Such materials can serve as key functional materials in fuel cells, electrochemical sensors and permeation membranes. In Sr(FexTi1–x)O3–
materials, which are good electronic and ionic conductors. Such materials can serve as key functional materials in fuel cells, electrochemical sensors and permeation membranes. In Sr(FexTi1–x)O3–![]() , the iron substitutes for Ti4+ partly in the oxidation state of Fe3+ and partly as Fe4+, the actual Fe3+/Fe4+ fraction depending on total iron concentration, oxygen partial pressure, and temperature. The charge compensation for Fe3+ occurs predominantly by the formation of mobile oxygen vacancies. For dilute Fe4+ centres (high spin d4 configuration) a Jahn-Teller distortion is predicted by quantum chemical calculations [1]. The formation of an iron impurity band occurs for iron concentrations higher than about 3-10% and represents a drastic change of the electronic structure [1]. The iron in metallic conducting SrFeO3 is known to have an undistorted octahedral coordination. The transition between these limiting cases is addressed in this study.
, the iron substitutes for Ti4+ partly in the oxidation state of Fe3+ and partly as Fe4+, the actual Fe3+/Fe4+ fraction depending on total iron concentration, oxygen partial pressure, and temperature. The charge compensation for Fe3+ occurs predominantly by the formation of mobile oxygen vacancies. For dilute Fe4+ centres (high spin d4 configuration) a Jahn-Teller distortion is predicted by quantum chemical calculations [1]. The formation of an iron impurity band occurs for iron concentrations higher than about 3-10% and represents a drastic change of the electronic structure [1]. The iron in metallic conducting SrFeO3 is known to have an undistorted octahedral coordination. The transition between these limiting cases is addressed in this study.
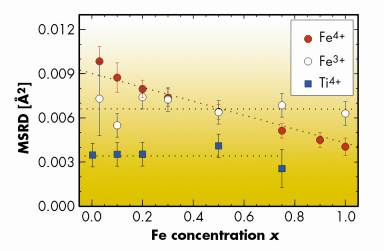
For each Fe concentration an oxidised (almost all Fe4+) and reduced (Fe3+) sample was investigated. Fe and Ti K-edge XAS spectra were recorded at beamline BM29. EXAFS show different local inter-atomic distances, for oxidised samples, Fe4+–O2– distances are smaller than half-lattice constants obtained by XRD. Splitting of the first Fe4+–O2– coordination shell was not observed directly. However, for oxidised samples, we observe an increase of the mean square radial distribution (MSRD) with decreasing x (Figure 105). For low x, MSRD of Fe4+ is even larger than that of Fe3+ for which oxygen vacancies are present. These findings also remained in low temperature measurements proving a strong static disorder. All these observations can be explained plausibly by a local Jahn-Teller distortion around dilute Fe4+ centres.
 |
|
Fig. 105: Concentration dependence of the MSRD for Fe4+–O2– and Ti4+–O2– bonds (oxidised samples) and Fe3+–O2– (reduced samples) in the first coordination shell, all at room temperature. Dotted lines are guides for the eye. |
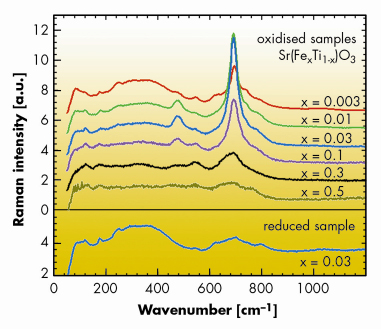
Raman spectra of oxidised samples show a new peak in the otherwise Raman-inactive cubic perovskite structure, Figure 106. This indicates a local symmetry breaking which is inseparably related to the presence of Fe4+, and thus strongly suggests a Jahn-Teller distortion (Note that the reduced samples, in spite of oxygen vacancies present, exhibit no Raman peak). The Raman peak decreases with increasing iron concentration at similar x where the iron impurity band starts to form, which is consistent with the absence of the Jahn-Teller distortion for SrFeO3.
 |
|
Fig. 106: Raman spectra for oxidised Sr(FexTi1–x)O3. The spectra are scaled to comparable intensity in the 200-400 cm–1 range and shifted upward for clarity. Bottom panel: reduced Sr(Fe0.03Ti0.97)O2.985 sample. |
Although none of the individual observations alone gives the final proof of a Jahn-Teller distortion around Fe4+ ions, the combination of results obtained by XAS, especially the iron concentration dependence of the Fe4+–O2– MSRD, and Raman spectroscopy strongly supports its presence, most pronounced for x ~ 0.03 and decreasing for higher iron concentrations. The decrease of the Jahn-Teller effect with increasing x can be understood qualitatively by the change in the electronic structure of the materials from insulator to metal. A quantitative modelling of the variation of the Fe4+–O2– MSRD and the intensities of the Raman lines remains a challenging theoretical problem.
References
[1] R.A. Evarestov, S. Piskunov, E.A. Kotomin, G. Borstel, Phys. Rev. B 67, 064101 (2003).
Principal publication and authors
M. Vracar (a), A. Kuzmin (b), R. Merkle (a) J. Purans (b), E.A. Kotomin (a), J. Maier (a), O. Mathon (c), Phys. Rev. B 76, 174107 (2007).
(a) Max Planck Institute for Solid State Research, Stuttgart (Germany)
(b) Institute of Solid State Physics, Riga (Latvia)
(c) ESRF



