- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2007
- Surface and Interface Science
- Real-time observation of ultrathin oxide growth during alloy oxidation
Real-time observation of ultrathin oxide growth during alloy oxidation
Atomically-flat oxide films with homogeneous chemical composition are usually required for optimum performance of tunnelling magneto-resistance (TMR) devices which depends critically on the tunnelling barrier thickness and the interface roughness. One way of preparing thin epitaxial oxide layers with a thickness in the sub-nanometre regime and a well-defined interface to the substrate is the selective oxidation of alloys under controlled conditions. Therefore, a precise structural and chemical analysis of the oxide films is mandatory, as well as in situ characterisation of the oxide growth process, which is still poorly understood.
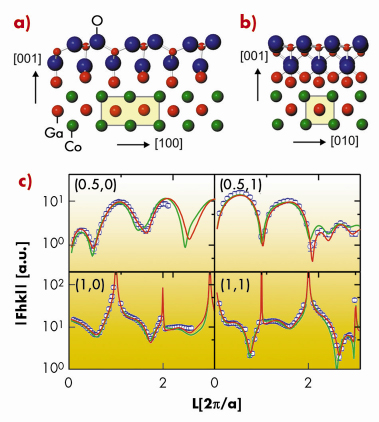
To unravel the atomistic structure of the well-ordered ultrathin epitaxial gallium oxide layer grown on the (100) surface of the inter-metallic B2-ordered alloy CoGa [1], we have adopted a multi-method approach where the results of high-resolution surface X-ray diffraction (SXRD) have been complemented by density functional theory (DFT) and core level spectroscopy (CLS) investigations. In addition, the initial stages of the atomic-scale oxide growth have been systematically studied using in situ SXRD. The X-ray experiment was conducted in an in situ chamber at the beamline ID32 using an incident photon energy of 20 keV. For the surface structure determination, an oxide layer was prepared by oxidation at 750 K and 5 x 10–7 mbar O2. A complete structural analysis was performed by measuring 16 symmetry non-equivalent surface rods and four independent crystal truncation rods.
The best fit and lowest DFT energy structural model (Figures 91a and 91b) was found to consist of an fcc-like oxygen ion double layer with gallium ions located in truncated octahedral and tetrahedral sites. At the interface, two more Ga ions occupy sites of the corresponding stacking sequence of bulk ß-Ga2O3. Figure 91c shows the experimental X-ray structure factors (open circles) together with those for the best fit (red curves) and the most stable DFT model (green curves). A remarkably good agreement between theory and experiment was observed, the average deviation between the X-ray and DFT positions within the oxide film being of 0.04 Å.
 |
|
Fig. 91: View of the refined structure of the Ga4O4 layer on CoGa(100) along: a) the (010) axis; (b) the (100) axis. (c) Experimental X-ray diffraction structure factors (open circles). The best fit and the most stable DFT model structure factors are indicated by the red and green curves, respectively. |
The onset of oxide formation was investigated in real time on the atomic length scale following the line profile and intensity on an oxide-sensitive reciprocal space position in situ, during oxidation. Figures 92a and 92b show the 2D diffraction pattern obtained from the reciprocal lattice intersection with the Ewald sphere at (1,1,1.2) for the surface firstly in its initial clean state and then after oxidation at 600 K and 10-6 mbar oxygen. The formation of two streaks during oxidation gives evidence for the growth of oxide islands with anisotropic shape in two domains. From the time-dependent diffraction signal, the average oxide island width and the time for the completion of one full layer was found to depend strongly on the oxygen partial pressure. This is interpreted as evidence for a transition from a predominantly on terrace nucleation of oxide islands at elevated pressures to a nucleation of oxide islands at step edges for pressures in the 10–8 mbar regime.
 |
|
Fig. 92: 2D diffraction pattern at (1,1,1.2): a) for the clean surface and b) after oxidation at 600 K and 10–6 mbar oxygen; (c) Scenario for anisotropic gallium oxide growth for one domain. |
An atomistic view of the scenario of the strongly anisotropic oxide growth was proposed, where oxygen is very likely supplied by dissociative chemisorption on the metal surface. The oxide grows much faster along the oxide (010) direction probably via the formation of parallel Ga-O-Ga chains, shifted relative to each other by half a substrate unit cell along the (010) direction (Figure 92c).
In conclusion, the surface gallium oxide film consists of an oxygen ion double layer which bears similarities with the bulk ß-Ga2O3 structure. An oxygen pressure dependent transition in the growth behaviour was observed, thus allowing the structural perfection of the oxide layer to be tailored by the appropriate choice of oxidation conditions.
Reference
[1] R. Franchy, Surf. Sci. Rep. 38, 195 (2000).
Principal publication and authors
A. Stierle (a), R. Streitel (a), P. Nolte (a), A. Vlad (a), I. Costina (a), M. Marsman (b), G. Kresse (b), E. Lundgren (c), J.N. Andersen (c), R. Franchy (d) and H. Dosch (a), New Journal of Physics 9, 331 (2007).
(a) Max-Planck-Institut für Metallforschung, Stuttgart (Germany)
(b) Computational Materials Physics and Centre for Computational Materials Science, Universität Wien (Austria)
(c) Department of Synchrotron Radiation Research, Lund University (Sweden)
(d) Institut für Grenzflächenforschung und Vakuumphysik, FZ Jülich (Germany)



