- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2006
- X-ray Absorption and Magnetic Scattering
- Time resolved in situ studies of oxygen intercalation into SrCoO2.5 performed by X-ray absorption spectroscopy and neutron diffraction
Time resolved in situ studies of oxygen intercalation into SrCoO2.5 performed by X-ray absorption spectroscopy and neutron diffraction
Fuel cells based on solid oxides allow the transformation of chemical to electrical energy using a large variety of different molecules. They represent a key technology in a modern and more environmentally friendly society. In recent years, great effort has been made in the optimisation of membranes characterised by good oxygen mobility. Unfortunately, good oxygen mobility is usually reached at high temperatures. For fuel cells, the operating temperatures are usually about 1000°C. Under such extreme conditions the mechanical and thermal stability of electrodes are significantly limited. One of the very few exceptions is the SrCoO2.5 system, which can be electrochemically oxidised via a reversible topotactic redox reaction, in an aqueous alkaline electrolyte at room temperature, yielding SrCoO3.0 as the final reaction product [1]. The reaction can formally be described by:
SrCoO2.5 + x O2- ![]() SrCoO2.5 + x + 2xe- (0 < x < 0.5) (1)
SrCoO2.5 + x + 2xe- (0 < x < 0.5) (1)
The electrochemical oxidation of the antiferromangetically ordered SrCoO2.5, with brownmillerite type structure, to the cubic ferromagnet SrCoO3, with perovskite structure, has been investigated in situ by neutron powder diffraction (NPD) as well as by XAFS spectroscopy, in specially designed electrochemical cells as a function of the charge transfer.
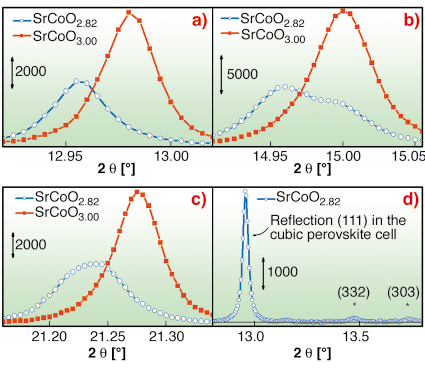
From the NPD experiments, two intermediate phases, SrCoO2.75 and SrCoO2.82±0.07, have been characterised. Superstructure reflections have been observed for the SrCoO2.82 phase only. To investigate the true symmetry of the intermediate phase, an electrochemically prepared sample of SrCoO2.82 has been measured ex situ on the high-resolution powder diffractometer at beamline BM01B. It was found that reflections, indexed in the averaged cubic perovskite cell as (h00) or (hh0), are clearly separated into two distinct diffraction peaks, whereas (hhh) reflections were unique, see Figure 107a-c, where the profiles of (111), (200), (220) reflections are reported and compared with that of the cubic SrCoO3 perovskite. This combined NPD and XRPD study represents the first observation of 3D oxygen ordering during an oxygen intercalation reaction. The SrCoO2.82 phase can be described as being in a tetragonal unit cell, related to the perovskite cell by a = b = 2a√2 and c = 2a. The structure of this intermediate phase confirms the strongly topotactic character of the oxygen intercalation reaction. Moreover, the observation of the reflections with XRPD, Figure 107d, at the same d –values found with NPD (2.48 Å and 2.08 Å) proves their non-magnetic origin.
 |
|
Fig. 107: High resolution XRPD collected at BM01B of cubic SrCoO3.00 (red) and SrCoO2.82 (blue) phases for the reflection profiles a) (111); b) (200); c) (220). d) the weak superstructure reflections (d = 2.48 and 2.08 Å), marked by the stars, are indexed in the I4/mmm space group. |
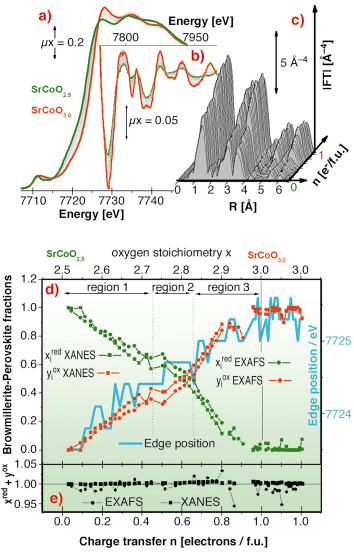
The SrCoO2.5 ![]() SrCoO3.0 oxidation has also been followed by in situ XAFS spectroscopy at the Co K-edge at BM29. Figures 108a and 108b report the evolution of the XANES, of the extended region of the absorption spectra, and of their k3-weighted FT. Figure 108d summarises the XAFS data, where all XANES (squares) and EXAFS (circles) spectra along the reaction have been simulated as a linear combination of the two reference spectra of the starting (SrCoO2.5) and final (SrCoO3.0) phases [2], without imposing the sum of the fraction to be equal to unity, see Figure 108e. From Figure 108d it emerges that the evolution of the Co valence state from formally +3 to +4 (XANES and edge shift) as well as the average Co local environment does not proceed continuously but gives the first evidence for the formation of O- species during an oxygen intercalation reaction, and more specifically for stoichiometries corresponding to SrCoO2.82±0.07.
SrCoO3.0 oxidation has also been followed by in situ XAFS spectroscopy at the Co K-edge at BM29. Figures 108a and 108b report the evolution of the XANES, of the extended region of the absorption spectra, and of their k3-weighted FT. Figure 108d summarises the XAFS data, where all XANES (squares) and EXAFS (circles) spectra along the reaction have been simulated as a linear combination of the two reference spectra of the starting (SrCoO2.5) and final (SrCoO3.0) phases [2], without imposing the sum of the fraction to be equal to unity, see Figure 108e. From Figure 108d it emerges that the evolution of the Co valence state from formally +3 to +4 (XANES and edge shift) as well as the average Co local environment does not proceed continuously but gives the first evidence for the formation of O- species during an oxygen intercalation reaction, and more specifically for stoichiometries corresponding to SrCoO2.82±0.07.
 |
|
Fig. 108: a) Evolution of the XANES; b) of the extended region of the absorption spectra; c) of their k3-weighted FT along the in situ SrCoO2.5 |
References
[1] J.C. Grenier, A. Wattiaux, J.-P. Doumerc, L. Fournes, J.-P. Cheminade, M. Pouchard, J. Solid State Chem. 96, 20-30 (1992).
[2] C. Lamberti, S. Bordiga, F. Bonino, C. Prestipino, G. Berlier, L. Capello, F. D’Acapito, F. X. Llabrés i Xamena and A. Zecchina, Phys. Chem. Chem. Phys., 5, 4502-4509 (2003).
Principal Publication and Authors
R. Le Toquin (a,b), W. Paulus (a), A. Cousson (b), C. Prestipino (c,d), S. De Panfilis (d) C. Lamberti (c), J. Am. Chem. Soc., 128, 13161-13174 (2006).
(a) Sciences Chimiques de Rennes, Université de Rennes 1 (France)
(b) Laboratoire Léon Brillouin, CEA-CNRS, Gif sur Yvette, (France)
(c) NIS centre of excellence University of Turin, and INSTM (Italy)
(d) ESRF (France)



