- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2004
- Structural Biology
- Crystal Structure of a Bacterial Sensor: Nitric Oxide Signalling Unveiled
Crystal Structure of a Bacterial Sensor: Nitric Oxide Signalling Unveiled
Nitric oxide (NO) has emerged as an important gaseous molecule that can influence a plethora of cellular functions [1]. Specifically, NO signalling is indispensable for the proper functioning of drugs like Viagra® and Cialis® used in the treatment of erectile dysfunction. In humans, NO is generated from the amino acid L-arginine by the enzyme nitric oxide synthase [2]. Physiological actions of NO are mediated by its heme protein sensor, soluble guanylyl cyclase (sGC), which gets activated upon binding NO up to 400-fold above basal level and catalyses the conversion of guanosine triphosphate to cyclic 3',5'guanosine monophosphate (cGMP) [3]. The primary function of sGC-derived cGMP is to trigger smooth muscle relaxation. Nearly 150 million men worldwide suffer from erectile dysfunction due to decreased NO production in the penile tissue and, therefore, fail to generate adequate amounts of cGMP. Both Viagra® and Cialis® enhance the erectile response by inhibiting the penile tissue-specific enzyme, phosphodiesterase-5, from breaking down cGMP. Although the basic mechanisms have been worked out, the structural basis for how NO activates sGC has remained elusive for nearly three decades.
Whereas the function of NO in humans is well established, its signalling role in bacteria is incompletely understood. Some bacteria generate NO from nitrite during a distinctive mode of respiration known as denitrification. Those lacking the machinery to produce NO find it to be toxic and utilise a molecular armamentarium to protect them from this poison. Indeed the century-old practice of nitrite curing of meats derives from the fact that NO is extremely toxic to Clostridium botulinum the etiological agent of Botulism. The molecular strategies utilised by C. botulinum to detect and avoid NO in its natural habitats are unknown.
We have succeeded in discovering the link between cGMP generation in humans and bacterial NO sensing mechanisms. We accomplished this by first proving the hypothesis that some bacteria harbour a NO sensing protein (SONO, sensor of NO) with striking resemblance to the NO-binding heme domain of sGC. Overexpression and purification of SONO resulted in a red-coloured protein containing a heme prosthetic group. Our work further demonstrated that SONO from C. botulinum has femtomolar affinity for NO. SONO purified from other bacteria (cyanobacteria and Vibrio cholerae), however, do not exhibit this extreme binding affinity. In light of its sequence similarity with sGC and specificity for NO, SONO is an excellent model system to understand the structural basis of gaseous messenger signaling.
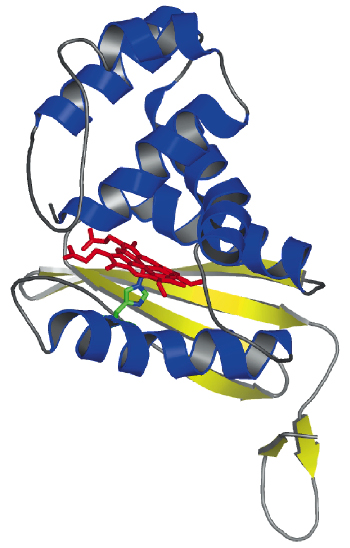 |
Fig. 69: Crystal structure of T. tengcongensis SONO. The heme group is shown as a stick model. |
We have determined the X-ray structure of Thermoanaerobacter tengcongensis SONO (39% sequence identity to C. botulinum SONO), at 2.5 Å resolution, using multiwavelength anomalous dispersion data from cubic crystals collected at the Fe K-edge at beamline ID29 of the ESRF. The three-dimensional structure of SONO is novel. It comprises an a-helical distal domain and ß-strand-rich proximal domain (Figure 69) with the heme nestled in between the two domains. The heme iron is coordinated by a highly-conserved histidine residue in the protein (Figure 70). Molecular recognition of heme is mediated by the electrostatic interaction between the propionate groups and conserved protein side chains. The structure also reveals a strategically located tyrosine side chain in the distal heme pocket for hydrogen bonding to NO (Figure 70). This residue is conserved in the C. botulinum protein and explains its strong binding affinity for NO. However, SONO from other bacteria and mammalian sGC have a hydrophobic distal pocket. Based on the high overall temperature factors for the distal domain, we posit that it undergoes conformational changes when NO binds to the heme.
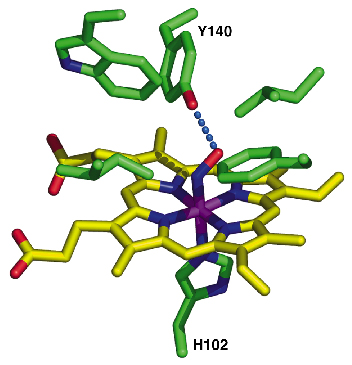 |
Fig. 70: A close-up view of the distal heme pocket highlighting the tyrosine side chain, which interacts and stabilises the heme-bound NO. |
Our structural analyses advances the conclusion that this domain's counterpart in mammalian sGC is poised to interact with the cyclase for signal transduction. By determining the crystal structure of a closely related bacterial sensor, we have gained insights into the inner workings of sGC and its ability to get activated by NO. Our structure will also help with identifying novel binding modes of activators that function independently of NO. Furthermore, our work provides clues to the 75-year old question: How does C. botulinum recognise and shun NO? In the grand scheme of things, this study has opened new vistas for additional research into signalling by gaseous messengers.
References
[1] F. Murad, Angew. Chem. Int. Ed. Engl. 38, 1856 - 1868 (1999).
[2] C.S. Raman, P. Martásek, and B.S.S. Masters The Porphyrin Handbook, 4, 293 340 (2000).
[3] D. Koesling, M. Russwurm, E. Mergia, F. Mullershausen, A. Friebe, Neurochem. Int. 45, 813 819 (2004).
Principal Publication and Authors
P. Nioche (a), V. Berka (a), J. Vipond (b), N. Minton (c), A.L. Tsai (a), and C.S. Raman (a), Science 306, 1550 1553 (2004).
(a) http://structural-biology.org; University of Texas Houston Medical School (USA)
(b) Health Protection Agency (UK)
(c) University of Nottingham (UK)



