- Home
- Users & Science
- Scientific Documentation
- ESRF Highlights
- ESRF Highlights 2001
- Chemistry
- From Fossilised Mastodon Ivory to Gemstone
From Fossilised Mastodon Ivory to Gemstone
In the Middle Ages, Cistercian monks created odontolite, a turquoise-blue gemstone, by heating fossilised mastodon ivory found in 13 - 16 million year old Miocene geological layers next to the Pyrenees. They thought they had produced the mineral turquoise because of the resemblance of odontolite with this semi-precious stone. Odontolite was therefore used for the decoration of medieval art objects such as the reliquary bronze cross shown in Figure 43.
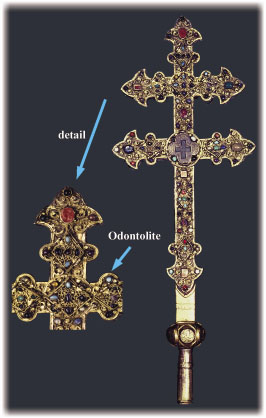 |
Fig. 43: Bronze reliquary cross of the "Real cross" from an atelier in Limoges dating from the XIIIth century with an odontolite sample (n° Cl.998), exhibited in the National Museum of the Middle Ages, Paris, © C2RMF. This is the first odontolite identified on a museum object by PIXE at the C2RMF. |
Fossilised ivory and its mysterious colour change upon heating have been investigated by several naturalists and gemmologists, among them Réaumur (1683-1757). Although vivianite, a blue-coloured iron phosphate, or copper salts were proposed to be the colouring phases, we found none of these minerals in odontolite. Our elemental and structural studies demonstrated that odontolite consists of fluorapatite, Ca5(PO4)3F, containing trace amounts of iron, manganese, barium, lead, rare earth elements and uranium and presenting crystallites of 100 to 500 nm in size. This crystal size is about 10 times larger than that of unheated fossilised ivory and suggests that odontolite was heated at about 600°C [1]. As potential colouring agents manganese and iron were then studied. Luminescence and optical spectroscopy permitted the exclusion of iron as a colouring ion and suggested that manganese ions could be responsible for the colouration of odontolite.
In order to investigate the origin of the heat-induced change of the colour of fossilised ivory, we used X-ray absorption spectroscopy to follow the changes in the local environment and the valence state of manganese on heating. XANES and EXAFS spectra were recorded at the K-edge of manganese impurities (200 to 650 ppm) in fluorapatite which is a strongly absorbing matrix at this energy (6.5 keV). Data were measured in the quick-scan mode by scanning the monochromator angle and the undulator gap on the ID26 beamline, devoted to the analysis of dilute samples. The excellent energy resolution (0.4 eV) achieved with the Si 220 monochromator on this beamline was necessary to measure the position and the intensity of the pre-edge structure of manganese. They indicate most clearly the changes in the structural environment and oxidation state of manganese. Unheated (white) fossil ivory shows Mn2+ ions in octahedral coordination (Figure 44a). By contrast, in the (turquoise-blue) fossilised ivory heated at 600°C and in the two odontolite samples, the major part of the manganese is in the 5+ oxidation state as indicated by the pre-edge structure observed at 6541.3 eV (Figure 44b-c). In addition, a comparison of the XANES spectra of heated fossilised ivory, odontolites and a reference synthetic Mn-chlorapatite (Figure 44e) indicates that the Mn5+ substitutes for P5+ at the tetrahedral sites [2]. In such a coordination Mn5+ ions give rise to an intense turquoise-blue colour [3].
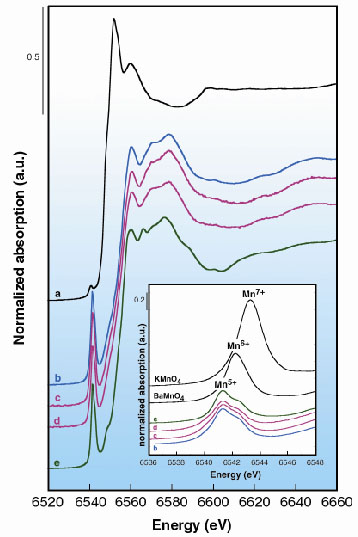 |
Fig. 44: Mn K-edge XANES spectra of: (a) fossilised ivory; (b) 600°C heated fossilised ivory; (c, d) turquoise-blue collection odontolites and (e) synthetic apatite Ba5(PO4)2.5(MnO4)0.5Cl taken as reference mineral with Mn5+ in tetrahedral coordination. Inset: Pre-edge structure of tetrahedral Mn5+ in (b) heated fossilised ivory; in (c, d) odontolites and in (e) synthetic Ba5(PO4)2.5(MnO4)0.5Cl, are observed at 0.9 eV and 1.9 eV below those of tetrahedral Mn6+ in BaMnO4 and Mn7+ in KMnO4, respectively. |
The transformation of white mastodon ivory into turquoise-blue odontolite involves thus two phenomena: (1) a fossilisation accompanied by an uptake of metal ions, specifically Mn ions (Mn2+), possibly by sorption on apatite crystallites; and (2) a deliberate heating process in air above 600 °C that oxidises Mn2+ into Mn5+, which substitutes for P5+ in the tetrahedral site of the apatite structure. This substitution occurs during the heat-induced crystal growth of apatite. Thus, we clearly demonstrated the origin of the colour change in fossilised ivory during heating using X-ray absorption spectroscopy and, in contrast to former hypotheses, that odontolite owes its turquoise-blue colour to traces of Mn5+ ions in a distorted tetrahedral environment of four O2 ions.
References
[1] I. Reiche, C. Vignaud, T. Calligaro, J. Salomon and M. Menu, Nucl. Instr. Meth. B, 161-163, 737 (2000).
[2] D. Reinen, H. Lachwa and R. Allmann, Z. anorg. allg. Chem., 542, 71 (1986).
[3] U. Oetliker, M. Herren, H.U. Güdel, U. Kesper, C. Albrecht and D. Reinen, J. Chem. Phys., 100, 8656-8665 (1994).
Principal Publication and Authors
I. Reiche (a, b), C. Vignaud (a, c), B. Champagnon (d), G. Panczer (d), C. Brouder (e), G. Morin (e), V.A. Solé (f), L. Charlet (g) and M. Menu (a), Am. Min., 86, 1519-1524 (2001)
(a) Centre de Recherche et de Restauration des Musées de France (C2RFM), CNRS- Ministère de la Culture, Paris (France)
(b) Present address: Rathgen-Forschungslabor SMPK, Berlin (Germany)
(c) Laboratoire de Physique des Liquides et Electrochimie, CNRS, Paris (France)
(d) Laboratoire de Physico-Chimie des Matériaux Luminescents, Lyon (France)
(e) Laboratoire de Minéralogie-Cristallographie de Paris (France)
(f) ESRF
(g) Groupe de Géochimie Environnementale - LGIT, Grenoble (France)



